The National Institutes of Health (NIH) Data Management and Sharing (DMS) Policy - mandating the submission of data management plans with grant proposals and the sharing of the project's scientific data - went into effect on January 25, 2023. The DMS Policy applies to all research, funded or conducted in whole or in part by the NIH, that results in the generation of scientific data.
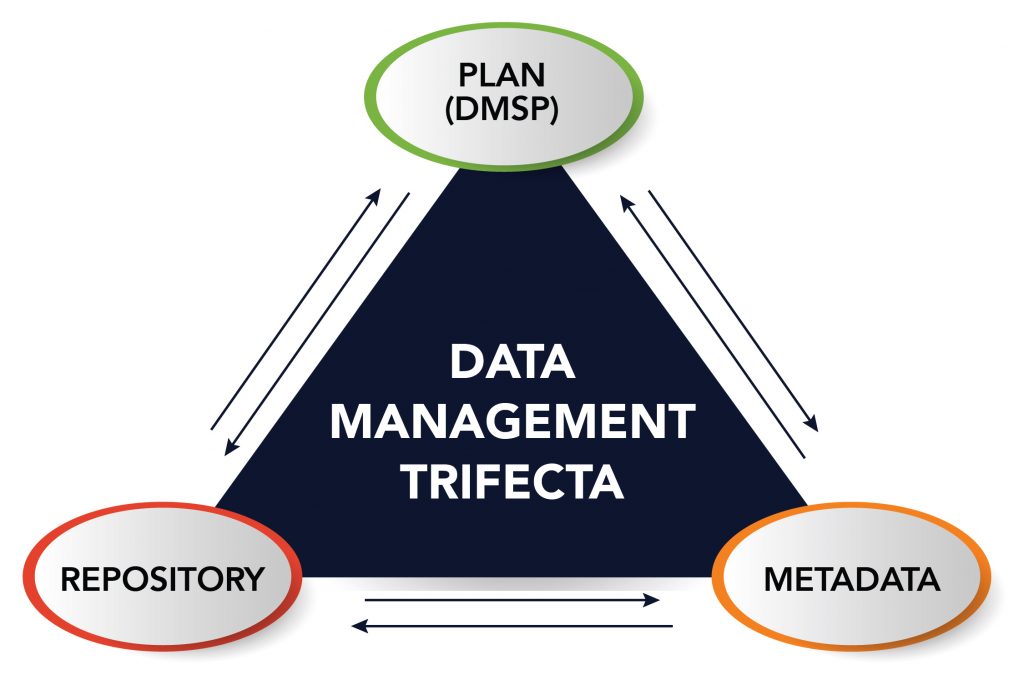
The NIH DMS Policy covers three components that must be addressed in order to comply with the policy: the Data Management and Sharing Plan (DMSP), Repository, and Metadata. The graphic below describes the connections between these three components.
The NIH Case for Data Sharing
- Speed up and improve science by testing the validity of research findings and enabling the vetting of the data and identifying sound data from fake data, or data with apparent errors; and, allowing the reuse of hard to generate data.
- Foster trust in publicly funded research/transparency
- Open new frontiers of discovery
- Advance the scientist’s career by increasing citations to papers for the data they shared. As an example, papers that provided a link to the data gained 25% more citations on average than those that did not, according to a 2020 study of more than 50,000 articles in the PLOS and BMC journals.
- Access to data from others saves time and money for smaller labs.
- Strengthen analysis through combined data sets.
- Pooling smaller data sets enables meta-analysis that may produce robust and intriguing findings. As an example - a study that gathered raw data from an array of animal studies conducted in the 1990s on treatments for spinal cord injury. The results from the individual studies were inconsistent and never published. But a 2021 analysis of pooled data from 1125 animals produced a significant correlation: Animals with blood pressure levels within a certain window during spine surgery fared better, a finding that held up in a clinical study.