Please note, the 780 and 880 Zeiss confocals are on inverted microscopes. Because of this, CCAM requires your samples to be sealed when mounted between a slide and cover glass. This will not only protect your sample but it will also protect our objectives and microscopes.
(Closed Caption Version (opens in a new tab))
Sample Thickness
Widefield microscope sections typically range from 4-10 microns. Confocal sections often range from 10-40 microns.
Coverslip
Please use a #1.5, or 170-micron thick, glass coverslip. All the Zeiss objectives are corrected to be used with this material and thickness.
Cell Fixation
There are a variety of protocols to maintain cellular structures when preparing fluorescent samples. Please refer to this white paper (opens in a new tab) for suggestions for preparing such samples.
Sealing Samples
Most commonly nail polish is used to seal slides, but it is toxic to live cells. Please refer to Coverslip Sealing (opens in a new tab) for a variety of options.
CoverGrip (opens in a new tab) is designed with ingredients that do not leach into an aqueous mounting medium and affect specimen fluorescence.
Mounting Media
Mounting media should have the same refractive index or be very close to Zeiss’ immersion oil which has a RI of 1.518.
It is highly recommended to use a mounting media that contains an anti-fade agent such as n-propyl gallate.
The type of mounting media you use will depend on which probes you are trying to visualize. Glycerol-based mounts, ProLong Diamond (opens in a new tab), are better for preserving structural information while mounts that harden can disrupt the cellular structure. You can seal these types of media before they harden and they will work equally as well and maintain the structures.
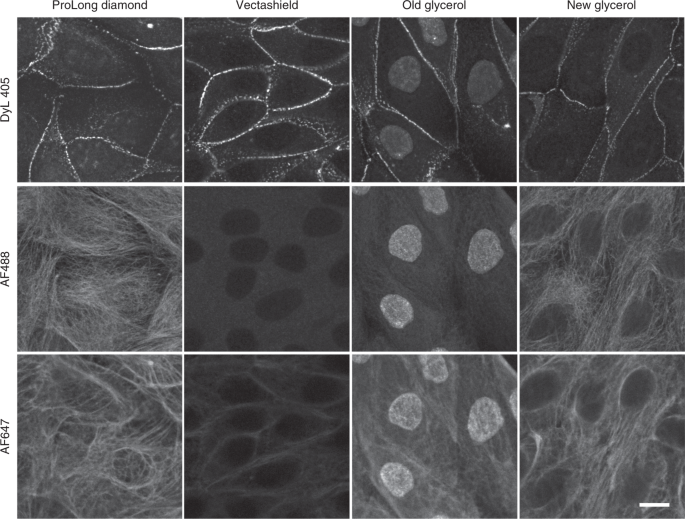
Mounting media can affect probes differently, so please make educated choices based on which probes you will be using. For example, Vectashield (opens in a new tab) is not compatible with Alexa 647 and other far-red dyes but works well with blue/green dyes. Likewise dated bottles of glycerol can result in unexpected staining results such as the nucleus appearing as background, as pictured below.
Jonkman, J., Brown, C.M., Wright, G.D. et al. Tutorial: guidance for quantitative confocal microscopy. Nat Protoc 15,1585–1611 (2020). https://doi.org/10.1038/s41596-020-0313-9 (opens in a new tab)
Commercial Resouces
ThermoFisher Mounting Media and Antifades (opens in a new tab)
Homemade Mounting Media
MOWIOL Mounting Media (opens in a new tab)
Jackson ImmunoResearch Anti-fade Mounting Medium (opens in a new tab)
A Word About DAPI
Do not use mounting media that contains DAPI since premixed mounting solutions with DAPI tend to increase background fluorescence. It is best to first stain with DAPI and then mount your sample separately.
Cell Fixation
ThermoFisher Scientific – 5 Steps to Fixed-Cell Imaging (opens in a new tab)