• General Questions
Who can use the UConn Health REDCap system?
User Eligibility
REDCap accounts are available to all University of Connecticut and UConn Health faculty, staff, and currently enrolled students for research-related purposes.
External user accounts will be given to non-UConn/non-UCH users via sponsorship by their UConn/UCH Principal Investigator (PI). On the application, they need to identify their UCH/UConn PI. We will then create an external account for them, which is linked to the PI’s account (thus, the PI must already have a REDCap account). External users will not have the ability to create new projects; they can only access projects to which they are formally added.
All REDCap users are required to use an institutional email address – NO personal email addresses (Gmail, Hotmail, etc.) are acceptable. No generic or multi-user accounts can be granted.
All REDCap users must complete CITI training (UConn / UCH) and any applicable HIPAA training. All REDCap users must also complete the Introductory Overviews, Basic Features & Functionality, and Project Types video training modules (approximately 1 hour) before using REDCap. All users are encouraged to review additional REDCap training materials as needed.
Once you have completed the training modules, to request access to REDCap, please complete and submit the REDCap Account Request.
*Please note that the REDCap Systems are not linked to each other, and separate accounts will be created for both the Development (test/sandbox) and Production system environments.
Usage Eligibility
REDCap usage is limited to non-commercial research purposes only. DHHS/Federal regulations define research as the systematic investigation, including development, testing, and/or evaluation, designed to develop or contribute to generalizable knowledge. Forms, questionnaires, or surveys for non-research purposes may be administered using an alternate service: Survey Tools & Assessment. REDCap may also not be used by for-profit entities, for-profit work, or in the conduct of clinical care; Nor can REDCap be used as a registry and/or repository. For more information, please see the REDCap User Agreement.
Project Eligibility
ALL projects must involve a PI who is a faculty member, employed and paid, by UConn Health or UConn. ALL projects must show proof of research use by providing the appropriate IRB documentation:
• a UConn/UConn Health approval letter or exempt determination letter, or
• a letter from the UConn/UConn Health IRB agreeing to rely on another IRB for oversight, or
• a Human Subjects Research Determination form signed by the UConn/UConn Health Human Subjects Protections Office indicating that the project is “NOT human subjects research” – the project MUST still be for research purposes only
The approved IRB documentation must be provided to the REDCap administrator along with the online Move to Production request form. The PI information listed on the request form must match the approved IRB documentation. The use of REDCap MUST be part of your approved protocol and data collection plan.
How do I get started?
How to Get Started
REDCap employs a streamlined process enabling you to rapidly develop a project:
- Learn more about REDCap and see if it will be an effective tool for your study. All project team members who will access and work in the UConn Health REDCap system must complete the Introductory Overviews, Basic Features & Functionality, and Project Types REDCap video training modules BEFORE requesting an account and using REDCap. These are the minimum requirements for training. All users are encouraged to view the additional REDCap training videos as needed.
- Request a REDCap account. REDCap accounts are available to all University of Connecticut and UConn Health faculty, staff, and currently enrolled students for research-related purposes. External Collaborator accounts will only be given to users via sponsorship by their UCH/UConn Principal Investigator (PI). Eligible users can register by completing and submitting a REDCap Account Application. Once we receive your completed application, we will create your account, and you will receive account activation instructions via email.
- Review the best practices and user guide.
- CREATE your project in the REDCap Development system. If you need help creating a practice project, please see our step-by-step Practice Project guide.pdf. If you are ready to create your own, make sure you check out the guides below which offer more depth and specific how-to’s.
- TEST your project thoroughly.
- DEPLOY your project from development to the production environment and start collecting your data. Real data can ONLY be collected in the production system.
How do I request a REDCap account?
Please complete and submit the REDCap Account Application
What if I forgot my password?
External (non-UCH) REDCap users are encouraged to set up a password recovery question when they create their account. In doing so, if you forget your password, you can quickly recover it by clicking the “Forgot Your Password” link on the REDCap login screen. If you are still unable to reset your password, please email the REDCap Administrator to request a password reset.
For UCH users, your account login is the same as your Institutional username and password and is not set by REDCap. As such, you will have to contact the IT helpdesk. The "Forgot Your Password" link is ONLY for non-UCH users.
How much experience with programming, networking and/or database construction is required to use REDCap?
No programming, networking or database experience is needed to use REDCap. Simple design interfaces within REDCap handle all of these details automatically.
It is recommended that once designed, you have a statistician review your project. It is important to consider the planned statistical analysis before collecting any data. A statistician can help assure that you are collecting the appropriate fields, in the appropriate format necessary to perform the needed analysis.
How much training is required to use REDCap?
All REDCap users must complete the Introductory Overviews, Basic Features & Functionality, and Project Types video training modules (approx. 1 hour) prior to using REDCap. These are the minimum training requirements. All users are encouraged to review additional REDCap training materials as needed:
- REDCap Training Videos
- Clinical Research Centers REDCap webpage
- Help & FAQ via the top of the main REDCap page
Make sure to also check out our Guides & How-to's
Is there any fees or costs associated with using the REDCap system?
The use of the development system is free of charge. A nominal fee of $150/per project will be charged when the project is moved to the production system. After the first year, all projects will be subject to a $75 annual fee
Additional support and add-on services are available at an hourly rate of $75. A minimum charge of 1/4 hour per service request applies. For these services, billing will be done at the end of the month the service was provided.
Invoices will be generated in our CORES billing system, and you will receive a notification from the system. If you have not been set up in our CORES billing system, you will need to register.
Payment is due within 60 days from invoice date If payment is not received your REDCap project/s will be deactivated.
Can I still maintain a paper trail for my study, even if I use REDCap?
You can use paper forms to collect data first and then enter into REDCap. All REDCap data collection instruments can also be downloaded and printed with data entered as a universal PDF file.
Can I link multiple projects that share common data?
Yes. As long as the projects have common fields such as demographics information to link to.
How do I access the collected data for advanced analysis at various points during the study?
Data from your study can be easily exported into Microsoft Excel and analysis packages such as SPSS, SAS, R, and STATA.
Does REDCap work on mobile devices like tablets and smart phones?
Yes! REDCap is entirely web-based; you can access it from any browser, anywhere on the planet, at any time.
No separate app, download, or software installation is needed. The view will automatically be optimized for whatever device is being used.
REDCap is compatible with (and can be used on) desktop computers, laptops, tablets (including iPads), smart phones (both Android and Apple), and any other device having an internet connection. There are no specific requirements to run REDCap on any of these devices and no known compatibility issues.
On most tablets, the default view is the same as a desktop computer. All features are available.
On most phones, the default view is REDCap Mobile - a view focusing on data entry. Not all features are available in this view. Any time you are on a smart phone, you can switch to desktop view at any time to get the full use of REDCap.
NOTE: We currently do not support REDCap mobile app feature that requires you to download the app from the app store. Only mobile browser based for now.
How should I cite REDCap for a publication?
Please note that any publication that results from a project utilizing REDCap should cite the following.
Study data were collected and managed using REDCap electronic data capture tools hosted at [YOUR INSTITUTION].1 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
1Paul A. Harris, Robert Taylor, Robert Thielke, Jonathon Payne, Nathaniel Gonzalez, Jose G. Conde, Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009 Apr;42(2):377-81.
What Boilerplate or IRB language should I use?
UCH REDCap is a HIPAA-capable web platform in that to be HIPAA-compliant it requires that users conduct certain practices, such as limiting access to and restricting export of high-risk data. As such, part of what makes UCH REDCap HIPAA-compliant is the appropriate data collection and management practices of UCH REDCap users - even those not collecting high-risk data. Please note, UCH REDCap is NOT a 21 CFR Part 11-compliant system and should NOT be used for studies reporting to the FDA (i.e., IND, IDE, abbreviated IDE, etc).
The REDCap platform is hosted on premise at UConn Health and managed by Academic IT Services and the Clinical Research Center. It is secured using the technical controls stipulated by HIPAA. These controls include (but not limited to): protection behind the firewall in the IT data center with controlled physical access, servers kept up to date with operating system and application patches and upgrades, role-based access to projects, data encrypted while in transmission, daily backup, and regular audits of system events and logs.
All REDCap data is stored on servers at UConn Health are protected by multiple firewalls. Remote access from outside the UConn Health network is provided securely over an encrypted web connection.
REDCap provides an intuitive interface for users to enter data and have real-time validation rules (with automated data type and range checks) at the time of entry. These systems offer easy data manipulation with audit trails and reporting and an automated export mechanism to common statistical packages (SPSS, SAS, Stata, R/S-Plus).
In addition, the REDCap application allows you to identify and protect fields that contain PII (Personally Identifiable Information) and/or Protected Health Information (PHI) data by employing fine-grained user rights that are set by the study administrator to control who can view, modify, or add data and/or forms. All activities are also system logged and are available in a full audit trail.
• Project Setup / Design
What types of projects can I create?
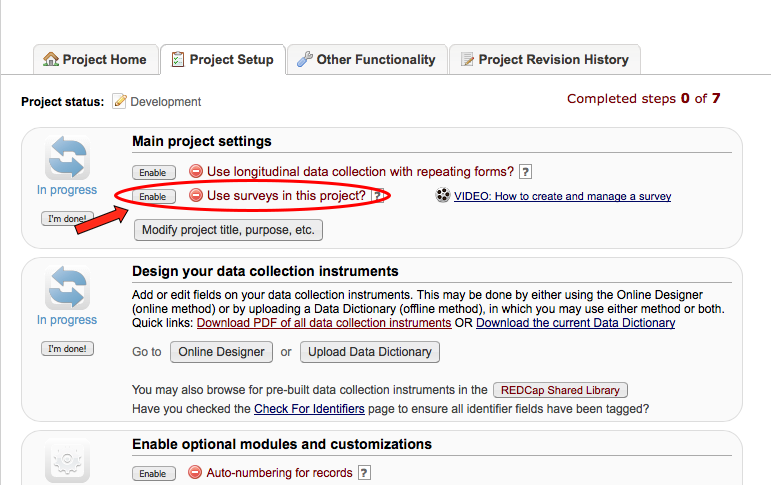
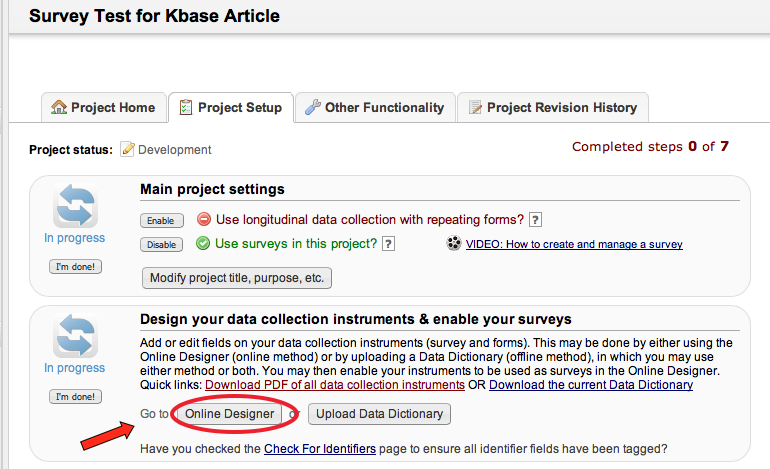
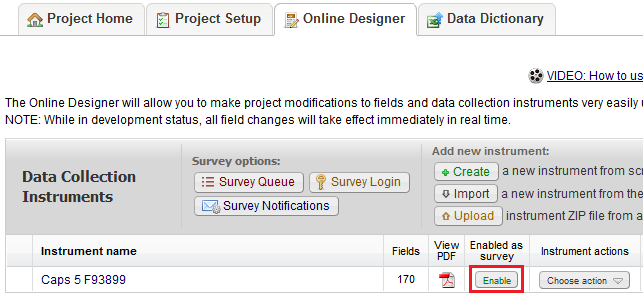
Multiple. You can create surveys as you would in SurveyMonkey and data forms, e.g. Case Report Forms (CRF); or a combination of both.
There are three project types available in REDCap:
-
Data collection performed only by study team using Data entry forms
- Data collection performed only by subjects / study participants using Surveys
- Screening phase before enrolling subjects using Survey + Data entry forms
There are two collection formats available for data entry forms:
-
Classic: one record per patient
-
Longitudinal: one record per patient per event, with the possibility of defining multiple arms
Please note: Your study materials dictate what and how data can be collected and how that data will be stored – this includes the usage of REDCap. Please refer to your approved study plan to determine if and how your project can utilize REDCap – this information can be found in the protocol, consent forms, IRB application, HIPAA authorization/waiver, etc.
What are the available field types?
A field is defined by the following attributes: type, label, name, validation, required, identifier, note.
Available field types:
- Text Box: single-line text box (for text and numbers)
- Notes Box: large text box for lots of text
- Calculated Field: perform real-time calculations
- Multiple Choice – Drop-down List: options for single answer
- Multiple Choice – Radio Buttons: options for single answer
- Checkboxes: checkboxes to allow selection of more than one option
- Yes – No: radio buttons with yes and no options; coded as 1, yes | 0, No
- True – False: radio buttons with true and false options; coded as 1, True | 0, false
- Slider/Visual Analog Scale: coded as 0-100
- File Upload: upload a document
- Descriptive Text (with optional image/file attachment): optional formatting feature
- Begin New Section (with optional text): starts new page
What is the difference between a data collection form and a survey?
Data Collection Forms:
- Must be filled out by a user who logs into REDCap.
- Simply add the users to your project and have them click on “Add/Edit Records”
- Advantages:
- Users entering data have the ability to see all data they’ve previously entered, edit previous responses, or check for updates.
- Users not only have access to data entry, but all other REDCap features (reports/exports, Training Videos, File Repository, Data Quality checks, etc.).
- Easier to troubleshoot errors because of the log that accompanies data entry.
- All entries are logged with a timestamp. REDCap tracks who entered data, any changes with old and new values, the IP address where it was entered, the date and time, etc. NOTE: This timestamp is not included in reports or exports though.
- User have the ability to give a form a status, marking it as “complete” or “incomplete” depending on what is most useful for workflow.
Surveys:
- Intended to be filled out participants (non-REDCap users)
- No login required.
- Responses are anonymous unless survey invitations are used and combined with a Participant Identifier (different from marking a field as an identifier in the Online Designer).
- Web interface has a sleeker design and can be customized with Logos and instructions.
- Advantages
- Survey responses are anonymous (can also be a disadvantage).
- All completed surveys have a date and timestamp that can be included in reports and exports.
- Easier to enter responses from a smartphone or tablet.
- No need for logging in or adding users.