The purpose of this module is to give the reader an overview of metastatic bone disease (MBD). Cancer that arises in an organ such as the lungs, breast, prostate, kidneys, thyroid and others and subsequently spreads to bone is termed metastatic bone disease (MBD). More than 1.2 million new cancer cases are diagnosed each year, and approximately 50 percent of these tumors can spread or metastasize to the skeleton. With improved medical treatment of many cancers, especially breast, lung and prostate, patients are living longer; however more of these patients develop bone metastases. After an overview of the manner in which cancer spreads from its site of origin to bone (pathophysiology), the natural history and work-up of this disease will be discussed, followed by a presentation of the surgical and non-surgical options.
Anatomy and Pathophysiology
How does cancer from an organ spread to bone? Two main theories are still entertained. In 1889, Sir James Paget, an English surgeon, developed his "seed and soil" theory by studying the medical records of 735 patients with breast cancer. The majority of metastases was noted to occur in the liver and brain. Dr. Paget realized there was a discrepancy between the blood supply and the frequency of metastasis in various organs. He then determined that local organ factors must favor implantation in specific sites. He did not feel that metastasis was related to the blood supply to a particular organ, as skeletal muscle and the spleen have a rich blood supply but are not frequent targets for metastasis. Dr. Paget felt that it was not simply that the cancer cells had the ability to survive and spread to a new site (the seed), but that the local environment had to be nurturing of further tumor growth (the soil). Only with both factors could successful metastatic spread occur.In contrast, James Ewing, M.D., an American pathologist, proposed his circulation theory in 1928. He proposed that tumors colonized particular organs due to the routes of blood flow carrying tumor cells away from the primary site. He thought organs were passive receptacles for tumor cells with no capacity to explain targeting to specific sites.The venous blood systems of the body, that part of the circulation responsible for returning the blood to the heart, includes a complex network that he guessed might account for the noted distribution of metastatic spread of cancer. The most common sites of metastasis for all cancers (not just those that also go to bone) are the lung and liver where the venous system is quite prominent. The venous system around the spine described by Oscar Batson, M.D., is proposed to explain why prostate cancer cells distribute preferentially to the pelvis and spine. This plexus involves a long set of veins that parallel the spinal column and allow back flow to bypass the more central system. Seventy-five years later, these two theories may not be mutually exclusive. The blood flow may help dictate the course of tumor cell travel, but inherent properties of the tumor cells and site of spread environment may facilitate growth.
Natural History
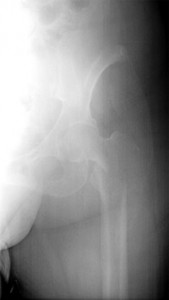
Reproduced with permission from Weber K., Lewis V., Randall R.L., Lee A.K., Springfield D.: An Approach to the Management of the Patient with Metastatic Bone Disease, in Helfet DL, Greene WB (eds): Instructional Course Lectures, Volume 53, Rosemont, IL, American Academy of Orthopaedic Surgeons, 2004, p.663-676.
The skeleton is the third most common site of spread of cancers that arise from organs or carcinomas. Metastases to the lung and liver are often not detected until late in the course of disease because patients experience no symptoms. On the contrary, bone metastases are generally painful when they occur. The vast majority of bone metastases originate from cancers of the breast, lung, and prostate, followed by the thyroid and kidney. The most common sites of spread in the skeleton include the spine, pelvis, ribs, skull, upper arm and leg long bones. Interestingly, these sites correspond to areas of bone marrow that demonstrate high levels of red blood cell production, the cells responsible for carrying oxygen to tissues in the body.
Patients with MBD require a team approach to care. A medical oncologist should work very closely with an orthopaedic surgeon very familiar with MBD, as well as a radiation oncologist. Pain management specialists and social workers are strongly recommended also. Scheduled follow-up or surveillance should be set up with each of these individuals as determined by the medical oncologist and/or surgeon.
Patients with MBD may have marked pain in the spine, pelvis or extremities because the bone is weakened by the tumor. This pain can be relieved with radiation treatments, pain medication or newer, minimally invasive surgical techniques such as radiofrequency ablation. Patients are sometimes at risk for developing a break in the bone that may leave them unable to walk or perform their usual daily activities. In such cases, surgery is usually necessary to repair the bone. The surgical techniques in these cases generally differ from those used for patients who sustain a broken bone from an injury (i.e., not because the cancer weakened the bone to the point where it breaks). Patients with cancer that has spread to the spinal bones may develop nerve damage that can lead to paralysis or loss of the use of the legs and/or arms. Sometimes the bone has not yet broken but is so weak that a break is imminent. Such scenarios are termed "impending fractures." Patients with impending or actual fractures may be forced to remain at bed rest for long periods of time, which can lead to possible chemical imbalances in the blood such as increased calcium levels (hypercalcemia). Anemia (decreased red blood cell production) is a common blood abnormality in these patients. The biggest concern for patients with metastatic bone disease is the general loss in their quality of life. Every cancer patient should discuss his or her risk for developing metastatic bone disease with their oncologist. Some cancers do not readily spread to bone while others do.
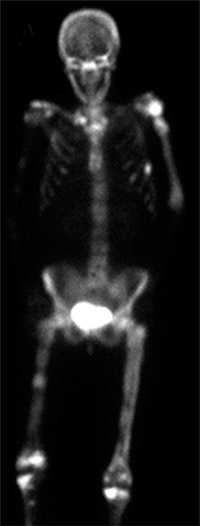
Reproduced with permission from Weber K., Lewis V., Randall R.L., Lee A.K., Springfield D.: An Approach to the Management of the Patient with Metastatic Bone Disease, in Helfet DL, Greene WB (eds): Instructional Course Lectures, Volume 53, Rosemont, IL, American Academy of Orthopaedic Surgeons, 2004, p.663-676.
The most common cancers that arise from organs and spread to bone include:
- Breast
- Lung
- Thyroid
- Kidney
- Prostate
If a cancer patient experiences any pain, especially in the back, legs and arms, they should notify their doctor immediately. Pain that occurs even without activity (i.e., walking or lifting an object) is particularly concerning. If the pain is getting worse, the patient should not delay in seeking out medical attention. It is important to have the physician ask the cancer patient important questions about their medical condition and how they feel. This is termed a medical history. The doctor will ask probing questions about the nature of any pain they are having, so it is best for the patient to think carefully about how they feel prior to the interview. After the medical history the physician will perform a physical examination concentrating on the painful areas. After the interview and physical examination, if the physician suspects that the patient may have metastatic bone disease, a radiographic examination will be ordered . Because some pain is referred from other areas (i.e., knee pain may be arising in the hip), the doctor may order radiographs that look at bone beyond the areas where the patient is experiencing the discomfort. The radiographic examination can tell the oncologist a great deal of information about if and how much the bone is involved. Sometimes, as in cancers that have spread to bone from the lung, thyroid, kidney and colon, the bone can be completely destroyed in a particular area (Figure 1). This is termed osteolytic. Alternatively, new bone can be formed in response to the cancer spread as is frequently seen in spread of prostate, bladder, and stomach cancer. This is termed osteoblastic. Breast cancer often behaves in a mixed osteolytic and osteoblastic manner. Osteolytic and osteblastic MBD occurs because the different cancer cells secrete factors that interact with the naturally occuring cells in the bone and cause either bone destruction, new bone formation or both.4
Surgical and Non-surgical Options
For the patient with an established MBD diagnosis, several treatment options must be considered. While radiation and/or surgery may be necessary, medical management may prove helpful as well. A class of medication called bisphosphonates has demonstrated a decrease in bone breaks due to breast and prostate cancer. These drugs interfere with the action of particular group of cells known as osteoclasts, whose job is to remove bone stores from bone. Earlier diagnosis and treatment for these particular cancers has also improved, so that patients are living longer. Patients with metastatic lung cancer to the skeleton tend to do less well. Patients who have bone metastasis originating from the kidney or thyroid have a unique set of challenges in that these tumors develop a new blood supply at the site of bone involvement. Furthermore, kidney cancer that has spread to bone is also very resistant to radiation treatments. Therefore, in some cases, surgical treatment has to be more aggressive to make sure that the site of bone involvement does not worsen.
Non-operative Treatment Benefits and Limits
Radiation for bone metastasis: Radiation can be a highly effective therapy in the treatment of MBD and is one of the most common modalities employed for treatment of symptoms in patients with incurable disease. Prior to initiating radiation therapy, the doctor and patient should have a clear understanding of the goals of therapy, whether it is to only minimize symptoms or pain/discomfort, or to completely eradicate disease in the area of concern. MBD is a systemic or body-wide problem, and radiation therapy is unlikely to be curative. Therefore, the physician must balance the potential benefits and risks of radiation for each patient afflicted with MBD.Radiation therapy kills tumor cells by its ability to generate molecules known as free radicals. These free radicals damage the DNA in cells that ultimately results in cell death. Several types of radiation therapy are available.
Local field radiation: Local field radiation is the most common type of radiation used to treat MBD. This treatment usually is directed at specific areas of the involved bone(s), with the target being the metastatic lesion and the immediate adjacent tissue. The treatment area may be expanded to cover entire bone segments or multiple bones depending upon the level of involvement. The primary goal is to achieve local pain relief with minimal side effects. However, there are instances when a larger treatment area may be needed in order to cover regions of potential metastatic involvement or to avoid future radiation of an adjacent area. The latter case is especially true when treating spine metastases. Matching a new treatment area onto a prior treatment area may result in damage to the spinal cord radiation tolerance at the junction of the two fields. This potential for "double treating" the junction would place the patient at increased risk for radiation-induced spinal cord damage. Therefore, several spinal segments (vertebrae) are usually included in the treatment field, although only one lesion is bothersome at the time of treatment. Local radiation therapy typically results in partial relief in more than 80 percent of cases and complete pain relief in 50 percent to 60 percent of cases. Variability in response rates depends on multiple factors including cancer type (i.e., breast and kidney) and the site of the lesion.41 Different cancers have different sensitivities to radiation. In general, lymphomas and multiple myeloma may respond faster and more completely to a given radiation dose, versus metastatic melanoma or renal cell carcinoma. The onset of pain relief usually occurs over the first 1 to 2 weeks, but maximal relief may take several months. Therefore, adequate pain medication must be maintained throughout the radiation treatment course.
Radiation treatment schedules: Fractionation refers to the how the radiation dose is divided. Standard fraction sizes for definitive treatment are typically 1.8 to 2.0 Gy per fraction given once per day. These smaller fractional doses are needed to reduce the chances of late radiation effects, especially when higher total doses are planned. When pain relief is the primary goal, as in MBD, the total dose is reduced, and therefore individual fraction sizes can be larger. Larger fraction sizes also allow more radiation dose to be delivered in a shorter time, which may be important for cancer patients with limited life due to advanced disease. One of the most common schedules used in the United States is 3 Gy x 10 fractions for a total dose of 30 Gy. The question of optimal pain relief fractionation schedule is a source of controversy. Proponents of shorter schedules or even single large palliative fractions quote studies showing equal efficacy in multiple endpoints between shorter versus longer treatment schedules. They suggest that quicker schedules are more appropriate for terminally ill patients with limited life expectancies. Conversely, advocates of longer treatment regimens contend that slightly protracted schedules allow higher total doses to be delivered and therefore result in higher rates of complete pain resolution and lower re-treatment rates. Some also maintain that the prediction of an individual patient's life expectancy is imprecise, and that fewer larger fractions leads to a higher risk of late radiation side effects in longer-term survivors. A recent meta-analysis by Wu and colleagues compared pain relief results from 16 randomized studies using various treatment schedules. They did not find a difference between any of the treatment regimens. The authors concluded that for pain relief, no regimen was superior to any other. They did find that patients treated with a single fraction did have higher rates of re-treatment to the original site, but the indications and efficacy of these repeat treatments could not be delineated. Although Wu and colleagues concluded that fractionation did not impact pain-related endpoints, they suggested that other palliative endpoints and treatment goals should be considered prior to choosing a treatment schedule. Radiation is effective in relieving pain secondary to bone metastases, but it does not have the ability to reverse an existing structural skeletal defect such as fracture. Radiation may halt the progression of bony destruction and allow healing of an impending pathologic fracture, but surgical fixation often remains the most expedient and effective solution. Following surgical treatment, local disease progression may lead to recurrent pain and failure of the fixation. Radiation therapy can be delivered in the postoperative setting to increase local tumor control after surgical stabilization. Data has shown that radiation following surgery improves patient function and decreases the rates of second orthopaedic procedures.
Hemibody irradiation and radioisotope therapy: Since most metastatic cancer patients have multiple lesions and approximately 75 percent of these patients will require additional radiotherapy within one year, large field radiation has been used in patients with widely metastatic disease. One such treatment involves hemibody irradiation (HBI). This treatment can be used to supplement local field radiation and may reduce the progression of widespread disease. HBI typically involves splitting the fields between the upper, middle, and lower body with partial blocking of the lungs to decrease the dose there. An alternative to HBI is radioisotope therapy. This "targeted" radiotherapy relies on the selective absorption of radiopharmaceuticals by areas of MBD and is easier to administer and to tolerate than HBI. Radioisotope therapy may be delivered intravenously and as an outpatient. It may be appropriate for situations of widespread metastases, when more traditional forms of radiation have been exhausted or when standard radiation techniques are not feasible due to surrounding normal tissue tolerances. Candidates for radioisotope therapy should have adequate bone marrow reserve (i.e., platelets > 100,000 and white blood cell count > 2500), because bone marrow suppression is a common side effect. Adequate kidney function is important, as the primary route of excretion for most radiopharmaceuticals is the kidney. Since the maximal effect may take weeks to months after administration, patients also should have an expected survival of > 3 months. Radioisotopes should not be first line therapy in the setting of advanced spinal disease in which the spinal cord is compressed because of their relatively slow response times. Examples of frequently used isotopes are strontium-89, samarium-153 and rhenium-186. Strontium-89 has a molecular structure similar to calcium and is absorbed into the bone matrix, especially in metastatic foci where active bone formation is occurring. The half-life is 50.6 days and it is primarily a short-range beta emitter. Response rates of 65 percent to 80 percent over a 3 to 6 month period have been reported with significant reductions in the rates of re-irradiation Newer isotopes such as samarium-153 are being used with success in similar situations. Samarium-153 has a shorter half-life (1.93 days) and a faster dose rate than strontium. It is a man-made isotope that is bonded to a phosphonate compound (EDTMP), which is preferentially incorporated into the bone. It emits radiation via beta and gamma decay and therefore can be imaged radiographically. Response rates have been comparable to strontium, but the shorter half-life can make it difficult to keep in stock. Rhenium-186 is a similar radioisotope with a half-life of 3.8 days that has shown comparable results to other isotopes in early studies.
Medical Treatment of Bone Metastasis: Medical treatment options for patients with skeletal metastases include chemotherapy, endocrine therapy, bone-specific therapy or a combination of treatments. Conventional chemotherapy has been effective in select cancer types such as lymphoma, small cell carcinoma, breast cancer and germ cell tumors. Breast and prostate cancers may respond well to endocrine therapies. Breast cancer patients with estrogen receptor positive tumors may have significant responses to tamoxifen therapy. Most prostate cancers are relatively sensitive to hormonal therapy with over 75 percent response rates to drugs that increase levels of lutenizing hormone-releasing hormone (LHRH) or gonadtropin hormone-releasing hormone (GnHRH). Total androgen blockade with drugs that increase LHRH or GnHRH combined with androgen receptor blockade seems to be more effective than single-agent therapy. Bisphosphonates bind preferentially to bone matrix and interfere with cells known as osteoclasts that are involved in the breakdown of bone. These drugs also promote osteoclast death and there is some suggestion that they may have similar direct effects on tumor cells. Bisphosphonates have been used with success to treat bone pain and elevated calcium levels in the blood (which can cause a variety of uncomfortable and dangerous health problems) in breast cancer, but they are most effective when used to supplement other systemic therapies. The benefit of the drug, particularly in advanced breast cancer and multiple myeloma, is primarily seen when it is administered intravenously rather than by mouth.
Surgical Intervention and Considerations
Surgery should be considered when an area of the skeleton is so involved that a break is highly likely or has already occurred. Important factors must be carefully assessed by the orthopaedic surgeon to determine whether a site of MBD is at significant risk of breaking: the way the area of involvement appears on the radiographs and how the patient feels. In the past, an area of bone was considered to be at risk for breaking if it was painful, larger than 2.5 centimeters, and involved more than 50 percent of the wall of the bone (cortex). In an attempt to quantify this risk, Dr. Mirels developed a scoring system based on the presence or absence of pain and the size, location and radiographic appearance of the area of concern. While other systems have been described and are not without merit, the Mirels classification is the system most widely used by surgeons today It is well established that patients undergoing surgical intervention to prevent a break do much better than those who require surgery to treat an actual break. They have shorter hospitalizations, are discharge to home more likely, return more quickly to previous activities, and have improved survival and fewer surgical complications. Elective surgical reinforcement of the area at risk also allows the medical oncologist and surgeon to coordinate operative treatment and systemic chemotherapy. The goals of surgical treatment in a patient afflicted with MBD and a bone at risk for breaking are to alleviate pain, reduce the need for pain medication, restore skeletal strength and regain functional independence. However, the decision to proceed with surgery is complex and must be individualized to each patient.
Operative Procedure
Surgical options depend upon the areas of involvement.
Treatment of Upper Extremity Metastasis: Twenty percent of bony metastases occur in the upper extremity (shoulder, arm and forearm) with approximately 50 percent in the humerus. Upper extremity metastases can result in significant functional impairment, hindering personal hygiene, independent ambulation, ability to use external aids, meal management and general activities of daily living. Treatment options include non-surgical management (radiation, functional bracing, and medications such as bisphosphonates), surgical stabilization and surgical removal and reconstruction. Patients not suitable for surgery are those with limited life expectancy, other severe medical problems, small lesions or tumors that can be treated with radiation alone. Radiation therapy can be administered alone or in combination with surgical management. The goals of surgery are stability, functional improvement and pain relief. The location and extent of the metastasis dictates the treatment option. Metastatic lesions of the collarbone (clavicle) and shoulder blade (scapula) are generally treated without surgery. Some cases however require surgical intervention.
Upper humeral (arm) lesions: Metastatic lesions to the upper humerus or arm bone may be addressed by a variety of techniques depending upon extent of involvement. Sometimes a portion of the upper arm and shoulder needs to be replaced with a large metal prosthesis (upper humeral prosthetic replacement). Generally it is only the arm side of the shoulder joint that is replaced when a patient has metastatic disease. The socket side of the joint is usually not involved. These surgeries are generally more complex than the shoulder replacements used for shoulder arthritis and patients often have worse function due to rotator cuff removal and reattachment to the metal prosthesis.
Humeral (arm) shaft lesions: Humeral shaft lesions are also treated with a variety of techniques although the joint generally does not need to be replaced. Polymethylmethacrylate (PMMA) or bone cement affords immediate stability, functional restoration and supplements poor bone quality.15 Humeral rods inserted down the central canal of the bone span the entire humerus and impart both mechanical and rotational stability. Sometimes the tumor will be removed if it is not sensitive to radiation, but often it is left in place because radiation treatment can kill the tumor after the bone has been stabilized. Segmental spacers offer a reconstructive option for treatment of shaft lesions. They are used in large defects and cases of failed prior surgery due to progressive disease. Segmental spacers can be used after resection of the metastatic lesion, minimizing blood loss in bloody lesions and often alleviating the need for postoperative radiation.
Open alignment and stabilization with plates and screws is another treatment option for humeral shaft lesions, although less commonly used than intramedullary fixation. The major drawbacks are the need for extensive exposure of the humerus and the inability to protect the entire bone. In the face of disease progression, the surgeon's inability to span the entire humerus puts the fixation at risk for failure.
Far humeral (arm) lesions (near the elbow): Far humerus lesions located above the elbow can be treated with a variety of techniques. Flexible nails offer the ability to span the entire humerus, excellent functional recovery, and preservation of the natural elbow joint. Elbow replacement may sometimes be necessary.
Forearm/hand lesions: Metastatic lesions below the elbow are rare. The most common primary tumors that metastasize to this location are lung, breast and renal cell carcinoma. Metastatic lesions in the radius and ulna can be treated with flexible rods, plates and screws or bracing. Lung cancer is the most common primary tumor that metastasizes to the hand.
Management of Lower Extremity Metastasis
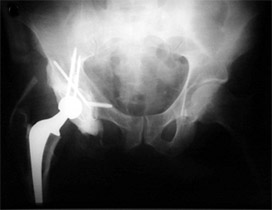
Patients with lower extremity metastasis have concerns related to pain and ability to walk. Fractures are more common, and the surgical techniques to stabilize the bones are becoming more standardized. Pelvic/acetabular (hip cup) lesions: The indications for surgical intervention in the pelvis are failed non-operative management, actual or impending fractures, and significant involvement of the hip joint cup (acetabulum), and other critical mechanical portions of the pelvis. If the acetabulum is involved, hip replacement (total hip arthroplasty) is generally necessary (Figure 3). Like the shoulder, these are more complicated than regular hip replacements. Surgically related problems are not infrequent, ranging between 20 percent to 30 percent of cases.
The femur (thighbone) is the most likely long bone to be affected by MBD. The upper third is involved in 50 percent of cases. Because the development of bone metastasis is a dynamic process, it is important to stabilize as much of the femur as possible.
Upper hip (femoral head/neck) lesions: Hip or femoral head and neck lesions, whether impending or actual, rarely heal; the procedure of choice is joint replacement. The indication for partial (hemiarthroplasty) versus total hip reconstruction is a function of acetabular or hip cup involvement.
Lower hip (peritrochanteric) femoral lesions: Placement of a metal rod down the central canal of the femur in this location has been more successful than screw and side plate implants . Sometimes, the area is so badly destroyed that the surgeon must replace the region with a special hip replacement, especially if the MBD is not sensitive to radiation treatment.
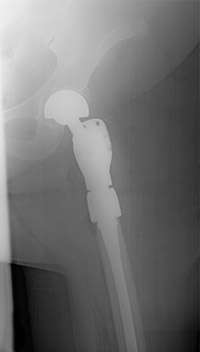
Reproduced with permission from Weber K., Lewis V., Randall R.L., Lee A.K., Springfield D.: An Approach to the Management of the Patient with Metastatic Bone Disease, in Helfet DL, Greene WB (eds): Instructional Course Lectures, Volume 53, Rosemont, IL, American Academy of Orthopaedic Surgeons, 2004, p.663-676.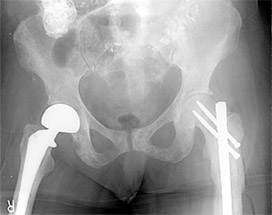
Reproduced with permission from Weber K., Lewis V., Randall R.L., Lee A.K., Springfield D.: An Approach to the Management of the Patient with Metastatic Bone Disease, in Helfet DL, Greene WB (eds): Instructional Course Lectures, Volume 53, Rosemont, IL, American Academy of Orthopaedic Surgeons, 2004, p.663-676.
Below the hip (subtrochanteric) femoral lesions: A substantial percentage of subtrochanteric pathologic fractures will not heal; therefore, fixation of fractures can lead to loss of fixation and/or hardware failure. The subtrochanteric area of the femur is subjected to forces four times to six times body weight. Screw and side plate constructs should not be considered in this area. Upper femoral replacement may be reserved for extreme cases where the bone is badly destroyed (Figure 4). For lesions where a break has not yet occurred but is likely, use of a metalic nail is the ideal modality (Figure 5).
Femoral shaft lesions: Placement of a metal nail down the central canal of the femur is the modality of choice for both actual and likely fractures.
Distal femoral (supracondylar) lesions: Lower end femur (supracondylar) lesions can be a challenge to treat secondary to multiple bone fragments and poor bone quality. Generally, good function can be obtained with a metallic implant but when the bone is badly destroyed, the end of the femur and the knee may need to be replaced. This form of knee replacement is usually more involved than the knee replacements for arthritis.
Shin bone (tibial) lesions: Metastasis to the shinbone (tibia) is far less common than the femur. When it does occur, it can be problematic, however. Conceptually, for upper shinbone lesions, similar principles should be employed to those used for the end of the femur: the upper tibia and knee usually need to be replaced. However, sometimes this is not necessary. For tibial shaft lesions, a metal rod is usually placed down the central canal of the bone. When the far end of the tibia is involved, various techniques can be employed, but generally plates and screws augmented with bone cement are advised.30
Foot lesions: Less than one percent of all bone metastasis involve the foot. The most common types are lung, kidney and colon. Treatment should be individualized and employ a combination of radiation therapy, orthotics and limited surgery.31
Spinal metastasis: MBD commonly localizes to the spine. Only the lung and liver are more frequently involved. Between 5 percent and 10 percent of all patients who have any metastatic cancer will have at least minor involvement of the spine. The majority of these patients will not need treatment.
Most cases of MBD to the spine do not need surgery. The presence of pain, the risk of developing a fracture, nerve compression and response to noninvasive or systemic treatments must be considered in the decision as to whether surgery should be performed. If the patient has pain but no nerve damage or risk of fracture, radiation treatment is preferred. If the patient has a tumor that is responsive to radiation, radiation can be used emergently even if there is neurologic compromise. The response is usually sufficiently quick that the risk of permanent nerve damage is no higher than that seen after surgery.
Over the past decade, minimally invasive or percutaneous techniques for MBD to the spine have been developed. Treatment of this type is used to control pain in patients who have developed certain types of fractures. One technique, vertebroplasty, involves percutaneous direct injection of bone cement or PMMA through the back. A more recent development, kyphoplasty, is a means of restoring normal spine alignment before injecting PMMA. The FDA has not approved PMMA for this indication, and therefore this is considered an "off label" use of PMMA. Nevertheless, surgeons at major cancer centers are using this technique with great success in select patients with MBD to the spine. Surgery is indicated for advanced cases of MBD to the spine. Patients with intermediate involvement who have continued pain after radiation may be indicated for surgical intervention.
Potential Operative Complications
Because patients afflicted with MBD are generally less healthy than the average patient undergoing orthopaedic surgery and the surgery is more involved, there is an increase in the routine risks of surgery, such as infection, bleeding, blood clotting, damage to nerves, etc. Accordingly, the patient, family, surgeon and oncologist must make a very careful and informed cooperative decision as to whether surgery should be undertaken. Advances in surgical techniques as well as radiation and medical therapies have significantly improved the quality of life for the individual suffering from cancer than has spread to the skeleton from its site of origin.