Assistant Professor (in Residence)
Department of Cell Biology
Contact
Phone: 860-679-2677
Email: egbert@uchc.edu
Office: E6029
UConn Health
263 Farmington Avenue
Farmington, CT 06030
Research Interests
My research interests are focused on physiological mechanisms of hormone signaling in the ovary, both within and between cells. During my postdoctoral research in the laboratory of Laurinda Jaffe, and now as an assistant professor-in-residence, I am studying the mechanisms by which luteinizing hormone (LH) acts on the outermost layer of granulosa cells to reduce cGMP levels within the follicle and cause oocyte meiotic resumption (Jaffe and Egbert, 2017). I am currently working on two projects that make use of novel mouse models:
Identifying the phosphatase(s) that mediate NPR2 dephosphorylation in response to LH
We have shown that LH triggers the rapid dephosphorylation and inactivation of the guanylyl cyclase, natriuretic peptide receptor 2 (NPR2), which reduces cGMP production in the granulosa cells (Egbert et al., 2014; 2021). This lowers cGMP throughout the follicle and oocyte within 20 min, leading to resumption of oocyte meiosis. NPR2 dephosphorylation is required for the normal time course of oocyte meiotic resumption (Shuhaibar et al., 2016), and this process requires the activity of one or more PPP-family phosphatases (Egbert et al., 2014) that remain unknown. To identify candidate phosphatase subunits that are rapidly regulated by LH signaling, we collaborated with the lab of Henning Urlaub (Göttingen, Germany) to assess LH-induced changes in protein phosphorylation of rat follicles using quantitative mass spectrometry (Fig. 1).
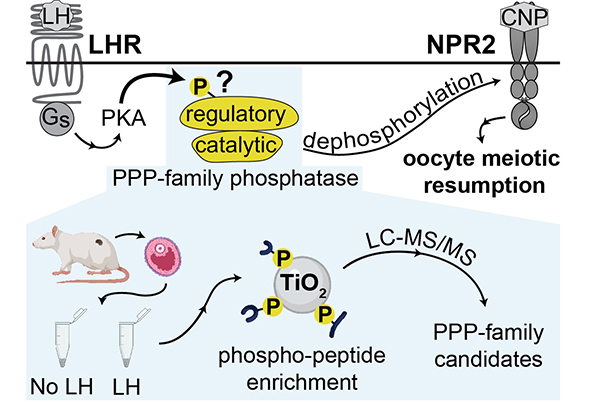
The PPP-family regulatory subunits with the largest LH-induced increases in phosphorylation are PPP2R5D (S53 and S566, as well as S81/S82), and PPP1R12A (S507). Mice in which phosphorylation of the four sites of PPP2R5D or the single site of PPP1R12A was prevented by mutating the serines to alanines did not affect NPR2 dephosphorylation or the timing of meiotic resumption in response to LH (2022 poster; Egbert et al., 2023). However, it is possible that the regulation of more than one phosphatase is required, and experiments are currently underway to test the combination of these mutations.
LH-induced cAMP dynamics within and between cells of the follicle
Using a mouse with a hemagglutinin (HA) epitope tag attached to the LH receptor (HA-LHR), it was recently revealed that LHR is expressed heterogeneously throughout the outer mural granulosa cells (Baena et al., 2020). In fact, only 10-50% of these cells are estimated to express LHR, which raises the question of how LH signaling can rapidly induce changes in cells throughout the follicle and oocyte. Although the spatiotemporal dynamics of cGMP in ovarian follicles have been investigated extensively, using a mouse line expressing the fluorescent sensor cGi500 (Shuhaibar et al., 2015), there is a gap in corresponding information for cAMP, due to lack of a mouse line with an optimal cAMP sensor. To study cAMP dynamics in intact follicles, we have made a mouse that expresses cAMPFIRE-M, a recently described FRET sensor for cAMP with excellent brightness and dynamic range (Massengill et al., 2022, Nat Methods 19:1461). Our goal in this project is to understand how LH-induced cAMP signaling is initiated and propagated throughout the follicle. We have used these mice to show that cAMP levels in the outer mural granulosa cells increase rapidly in response to LH, and that this elevation of cAMP spreads through gap junctions all the way to the oocyte. The transient increase in oocyte cAMP represents a barrier to meiotic resumption that had not previously been observed, and we are working to understand how cAMP falls again to allow meiosis to resume (see 2023 poster). In the future, we plan to test whether LH exposure initially causes only a subset of outer mural granulosa cells to elevate cAMP, as suggested by the heterogenous expression of LHR. We will also test whether cAMP diffuses from these LHR-expressing cells through gap junctions into adjacent cells that lack LHR. Because NPR2 is inactivated by cAMP signaling (Egbert et al., 2021, 2023), such a mechanism could explain the rapid and widespread cGMP decrease that is necessary for meiotic resumption of the oocyte.
These projects will investigate long-standing questions in membrane guanylyl cyclase regulation and cyclic nucleotide signaling, increasing our understanding of how the mid-cycle LH surge rapidly alters cyclic nucleotide levels in the follicle and oocyte to trigger timely oocyte meiotic resumption and release a fertilizable egg.
Recent Publications
Egbert, J.R., Silbern, I., Uliasz, T.F., Lowther, K.M., Yee, S.-P., Urlaub, H., Jaffe, L.A. 2023. Phosphatases modified by LH signaling in ovarian follicles: testing their role in regulating the NPR2 guanylyl cyclase. Biology of Reproduction (in press).
Egbert, J.R., Uliasz, T.F., Lowther, K.M., Kaback, D., Wagner, B.M., Healy, C.L., O’Connell, T.D., Potter, L.R., Jaffe, L.A., Yee, S.-P. (2022) Epitope-tagged and phosphomimetic mouse models for investigating natriuretic peptide-stimulated receptor guanylyl cyclases. Front. Mol. Neurosci., 15:1007026.
Cyclic AMP links luteinizing hormone signaling to dephosphorylation and inactivation of the NPR2 guanylyl cyclase in ovarian follicles. Biol Reprod. 104 (5):939-941.
Baena, V., Owen, C.M., Uliasz, T.F., Lowther, K.M., Yee, S.-P., Terasaski, M., Egbert, J.E., and Jaffe, L.A. (2020). Cellular heterogeneity of the LH receptor and its significance for cyclic GMP signaling in mouse preovulatory follicles. Endocrinology 161 (7):bqaa074.
Egbert, J.R., Fahey, P.G., Reimer, J., Owen, C.M, Evsikov, A.V., Nikolaev, V.O., Griesbeck, O., Ray, R.S., Tolias, A.S., and Jaffe, L.A. (2019) Follicle-stimulating hormone and luteinizing hormone increase Ca2+ in the granulosa cells of mouse ovarian follicles. Biol. Reprod., 101(2), 433–444.
Egbert, J.R., Yee, S.-P., and Jaffe, L.A. (2018) Luteinizing hormone signaling phosphorylates and activates the cyclic GMP phosphodiesterase PDE5 in mouse ovarian follicles, contributing an additional component to the hormonally induced decrease in cyclic GMP that reinitiates meiosis. Develop. Biol. 435:6-14.
Jaffe LA and Egbert JR. (2017) Regulation of Mammalian Oocyte Meiosis by Intercellular Communication Within the Ovarian Follicle. Annu. Rev. Physiol. 79: 237-260.
Egbert JR, Uliasz TF, Shuhaibar LC, Geerts A, Wunder F, Kleiman RJ, Humphrey JM, Lampe PD, Artemyev NO, Rybalkin SD, Beavo JA, Movsesian MA, Jaffe LA. (2016) Luteinizing Hormone Causes Phosphorylation and Activation of the cGMP Phosphodiesterase PDE5 in Rat Ovarian Follicles, Contributing, Together with PDE1 Activity, to the Resumption of Meiosis. Biol. Reprod. 94(5) :110. Supplement.pdf.
Egbert JR, Shuhaibar LC, Edmund AB, Van Helden DA, Robinson JW, Uliasz TF, Baena V, Geerts A, Wunder F, Potter LR, Jaffe LA. (2014) Dephosphorylation and inactivation of NPR2 guanylyl cyclase in granulosa cells contributes to the LH-induced decrease in cGMP that causes resumption of meiosis in rat oocytes. Development. 141(18):3594-604.
