Academic medical centers operate in a complex and highly regulated environment governed by a plethora of federal and state laws and regulations. As such, the establishment of an effective compliance and ethics program is necessary to adequately and responsibly safeguard institutions and carry out their various ethical, legal and fiduciary responsibilities. As a condition of participation, many federal programs, such as Medicare and Medicaid, mandate comprehensive compliance and ethics programs.
In addition to meeting its legal requirements of having an effective compliance and ethics program, the Office of Healthcare Compliance and the Office of University Compliance work together to prevent and detect compliance violations, mitigate damaging and costly risks, and promote a culture of compliance and ethics across all the University’s campuses.
Summarized herein, are some of the foundational elements of an effective compliance program, which are based on Chapter 8 of the Federal Sentencing Guidelines for Organizations.
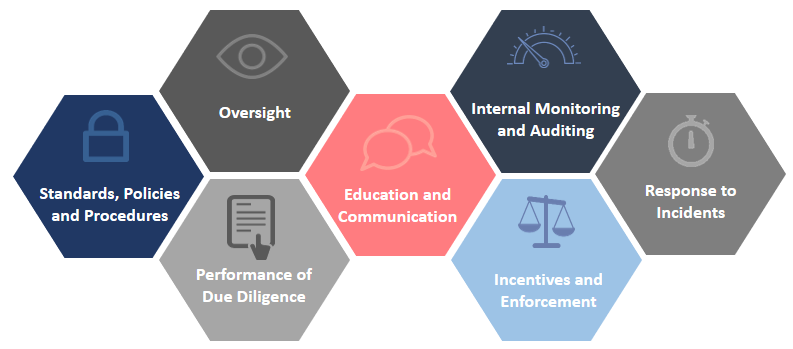
1. Standards, Policies, and Procedures – Establish standards and procedures to prevent and detect criminal conduct and facilitate compliance.
2. Oversight and Accountability – Ensure high level personnel exercise reasonable oversight with respect to the implementation and effectiveness of the compliance and ethics program, and are knowledgeable about the content and operation of the program. Specific individual(s) within high-level personnel (e.g., Chief Compliance Officer) shall be assigned overall responsibility for the compliance and ethics program.
3. Performance of Due Diligence – Use reasonable efforts not to include any individual who the organization knew or should have known (through due diligence) to be engaged in illegal activities or conduct inconsistent with an effective compliance and ethics program.
4. Education and Communication – Establish effective training and education programs and take reasonable steps to communicate standards, procedures, roles and responsibilities, as well as other aspects of the program to members of the institution, including the governing authority, high level personnel, substantial authority personnel, organization employees, and the organization’s agents (as appropriate).
5. Internal Monitoring and Auditing – Take reasonable steps to ensure the compliance and ethics program is followed by monitoring and auditing to detect criminal activity, non-compliance, and program effectiveness, as well as establish and publicize a mechanism that allows for anonymous and confidential reporting without fear of retaliation.
6. Incentives and Enforcement – Consistently promote and enforce standards through appropriate incentives, as well as disciplinary measures for engaging in criminal conduct and/or failing to take reasonable steps to prevent or detect criminal conduct.
7. Response to Incidents – Take reasonable steps to appropriately respond to criminal conduct/non-compliance, and to prevent further similar conduct from occurring in the future, including any modifications to the organizations compliance and ethics program.
 We are pleased to announce that Alyssa Cunningham has been appointed to the role of Assistant Vice President for Healthcare Compliance and Privacy. Alyssa is an experienced lawyer with 14 years of concentrated health care law, compliance and privacy experience, who joined UConn Health’s Office of the General Counsel in 2017. With the health care regulatory environment constantly changing, Alyssa’s legal experience, knowledge of UConn Health, and expertise in the areas of health care compliance and privacy will prove extremely valuable.
We are pleased to announce that Alyssa Cunningham has been appointed to the role of Assistant Vice President for Healthcare Compliance and Privacy. Alyssa is an experienced lawyer with 14 years of concentrated health care law, compliance and privacy experience, who joined UConn Health’s Office of the General Counsel in 2017. With the health care regulatory environment constantly changing, Alyssa’s legal experience, knowledge of UConn Health, and expertise in the areas of health care compliance and privacy will prove extremely valuable.

