Professor and Chair
Professor and Chair
Department of Cell Biology
Contact
Phone: 860-679-2661
Email: ljaffe@uchc.edu
Office: E6032
UConn School of Medicine
263 Farmington Avenue
Farmington, CT 06030
Curriculum Vitae
Research Interests
Research in the Jaffe lab concerns the physiological mechanisms that regulate the oocyte cell cycle, ovulation, and fertilization. Currently, our studies are focused on regulation of meiosis and ovulation in mammalian ovarian follicles by luteinizing hormone.
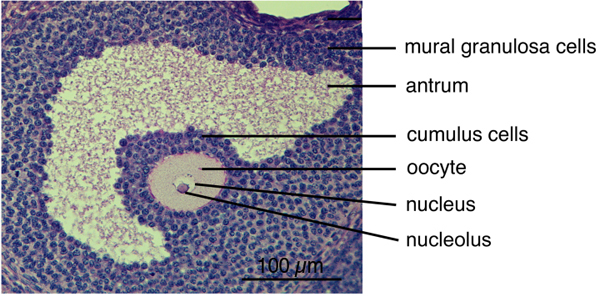
Meiosis prepares the oocyte for fertilization, by reducing the number of copies of each gene from two to one, such that the female and male genomes from oocytes (eggs) and sperm can combine to make a new individual. In mammalian oocytes, meiosis begins during embryonic development of the mother, and then arrests in prophase for a prolonged period. Much of the process of meiosis occurs within a spherical complex of somatic cells, called granulosa cells, that make up a follicle; the outer granulosa cells are called mural granulosa, and the granulosa cells closest to the oocyte are called cumulus (see photo below). During each reproductive cycle, a group of follicles grow to a stage at which luteinizing hormone from the pituitary can act on the mural granulosa cells, to cause resumption of meiosis and ovulation of a mature fertilizable egg.
The follicle functions as a coordinated system in which processes in the granulosa cells, as well as processes in the oocyte itself, regulate meiotic progression in the oocyte.
Work in the Jaffe lab has established that a Gs G-protein and an associated receptor, GPR3, both located in the oocyte, contribute to maintaining meiotic prophase arrest in mouse oocytes (Mehlmann et al., 2002; 2004). The activity of the Gs G-protein leads to production of cyclic AMP in the oocyte, and high cAMP keeps the cell cycle arrested during storage in the ovary .
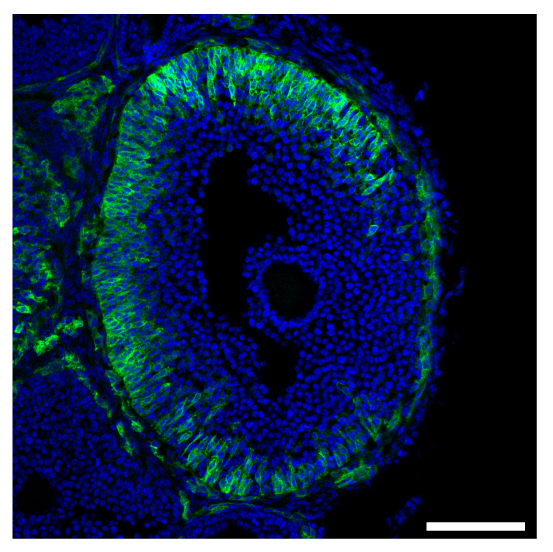
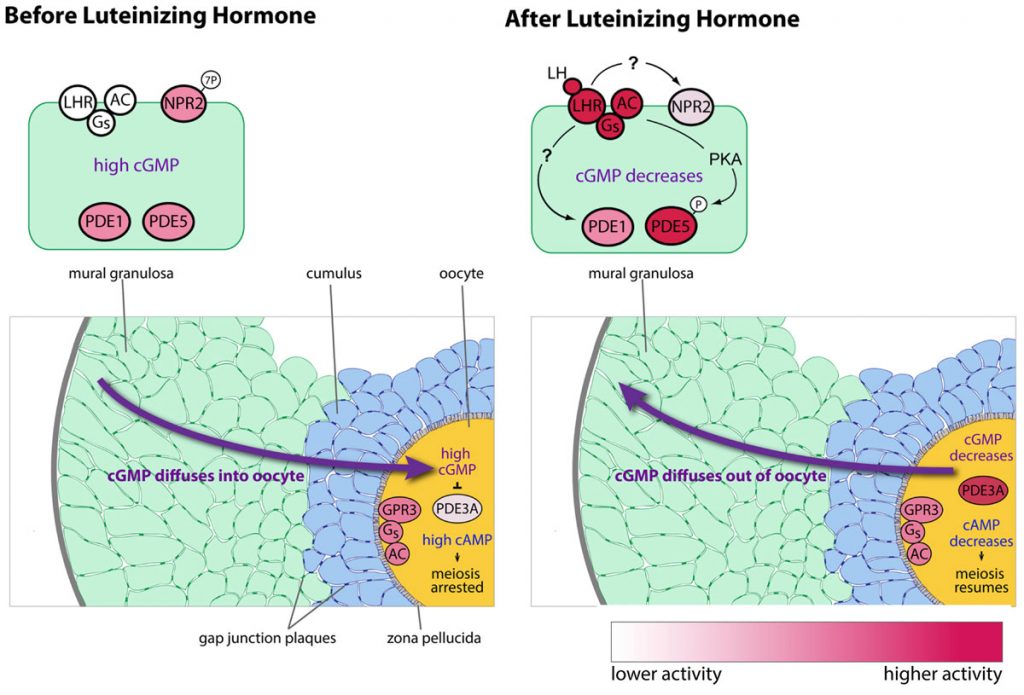
We have also determined that the granulosa cells of the follicle contribute to maintaining meiotic prophase arrest by regulating the hydrolysis of cAMP in the oocyte. This regulation involves another cyclic nucleotide, cyclic GMP, which diffuses from the granulosa cells into the oocyte, where it inhibits a cAMP phosphodiesterase, PDE3A, and thus maintains the high cAMP that maintains meiotic arrest (Norris et al., 2009). Then in response to luteinizing hormone (LH), which acts on a G-protein coupled receptor in the mural granulosa cells, the signaling system in the follicle switches, such that cAMP in the oocyte decreases and meiosis proceeds. This occurs primarily because LH signaling lowers cGMP in the granulosa cells (Norris et al., 2009). The LH receptors are located in a heterogeneous population of cells in outermost region of the mural granulosa (Baena et al. 2020; Owen and Jaffe, 2024).
Cellular heterogeneity of the expression of the LH receptor, as detected in an ovarian follicle from a mouse in which the endogenous LH receptor has an HA epitope tag added.
The cGMP decrease in the granulosa cells begins within less than a minute after LH application (Shuhaibar et al., 2015). We can monitor this change using confocal microscopy of follicles from mice expressing a FRET sensor for cGMP, cGi500. The cGMP sensor mice were made by the lab of our collaborator, Robert Feil (University of Tubingen).The LH-induced cGMP decrease occurs in a wave progressing inwards. In the outer granulosa cells, where the LH receptors are located, cGMP decreases to half of its plateau value within ~3 minutes. cGMP in the oocyte falls to half its plateau value within ~10 minutes, as a result of diffusion into the large volume of the surrounding granulosa cells, by way of gap junctions (Shuhaibar et al., 2014).
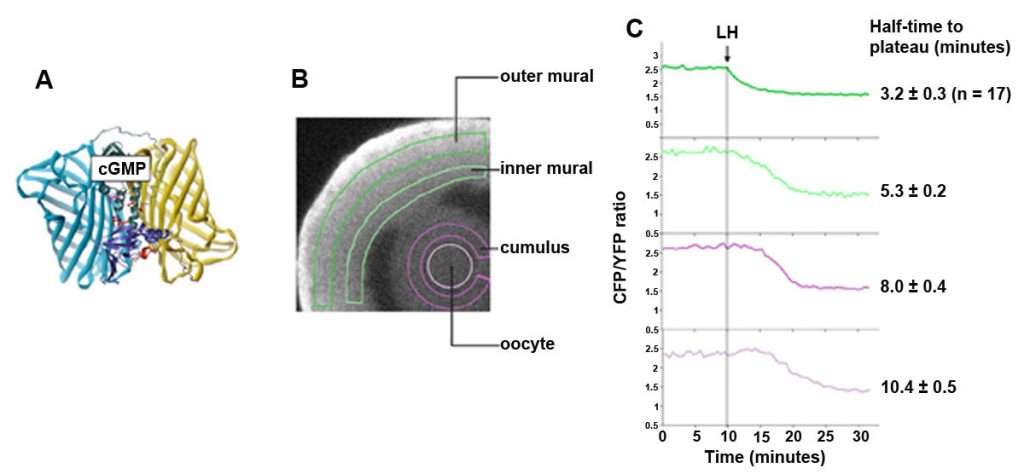
Cyclic GMP is produced in the mural granulosa and cumulus cells by the guanylyl cyclase natriuretic peptide receptor 2 (NPR2), which is activated by C-type natriuretic peptide (CNP); CNP is produced by the mural granulosa cells (work from the lab of John Eppig ; see Zhang et al., 2010, Science 330, 366-369). Our work, in collaboration with the lab of Lincoln Potter (University of Minnesota), has shown that LH signaling reduces cGMP in part by reducing its production, through rapid dephosphorylation and inactivation of the guanylyl cyclase NPR2 and a slower reduction of its agonist CNP (Robinson et al., 2012; Egbert et al., 2014; Shuhaibar et al, 2016). LH exposure also results in rapid phosphorylation of the phosphodiesterase PDE5. This is accompanied by a rapid increase in PDE5 activity, indicating that in parallel with decreasing cGMP production, LH signaling increases cGMP hydrolysis (Egbert et., 2014; Egbert et al., 2016, 2018). As a result, cGMP decreases in the mural granulosa cells, and then in the oocyte, leading to the resumption of meiosis.
Ongoing Research
LH signaling reinitiates meiosis by activating protein kinase A (PKA), dephosphorylating NPR2, and lowering cGMP in the granulosa cells and oocyte, thus lowering cAMP in the oocyte. But what is the link between PKA and NPR2 dephosphorylation? Does PKA signaling activate a phosphatase? Or inactivate a kinase? Which phosphatase? Which kinase?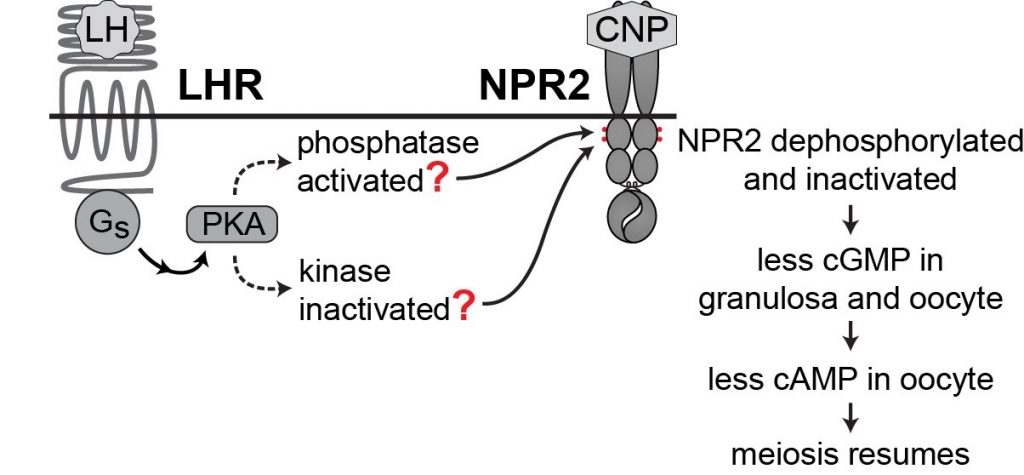
LH reinitiates meiosis in the oocyte by way of its Gs-linked receptor in the mural granulosa cells to increase cAMP in these cells. We have measured the kinetics of cAMP changes in each region of the follicle, using mice expressing the cAMPFIRE-M sensor in all cells. cAMP stays high for hours in the mural cells, but only transiently in the oocyte. See Poster
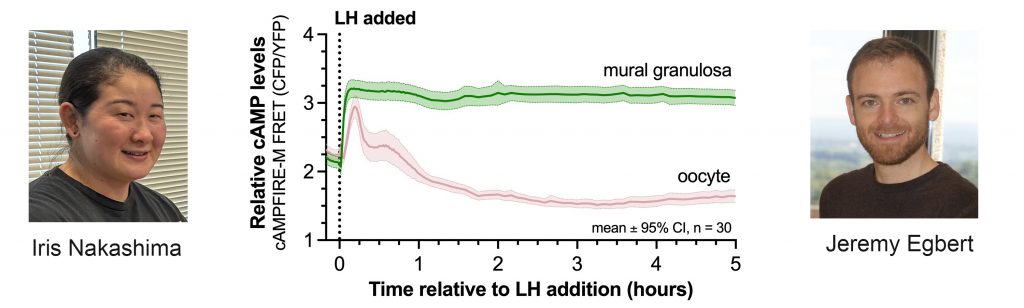 With gap junctions open, why doesn't the prolonged cAMP elevation in the granulosa cells counteract the cAMP decrease in the oocyte? This problem is alleviated because at 30 minutes after LH is applied, after cGMP in the oocyte has decreased, gap junctions between granulosa cells close.
With gap junctions open, why doesn't the prolonged cAMP elevation in the granulosa cells counteract the cAMP decrease in the oocyte? This problem is alleviated because at 30 minutes after LH is applied, after cGMP in the oocyte has decreased, gap junctions between granulosa cells close.
Gap junction closure results from EGF receptor ligand release from the outer mural granulosa cells in response to LH signaling, causing connexin phosphorylation. Blocking EGF receptor signaling (AG1478) inhibits gap junction closure (Norris et al., 2010).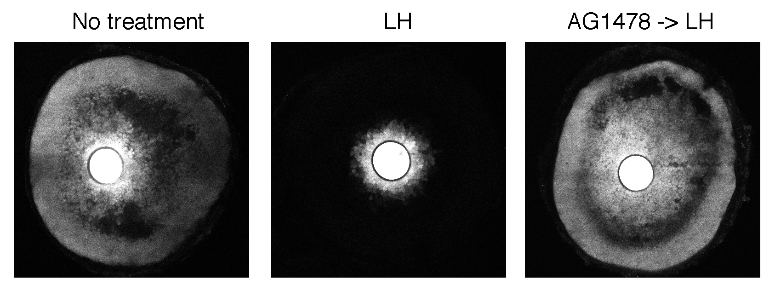
Gap junction closure accelerates the decrease in oocyte cAMP that triggers meiotic resumption in response to luteinizing hormone.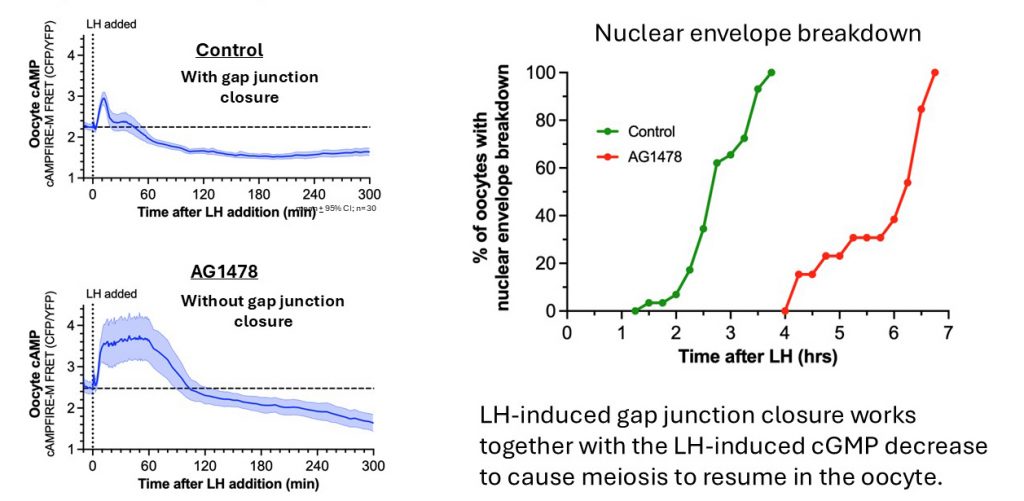
New Directions: Ovulation
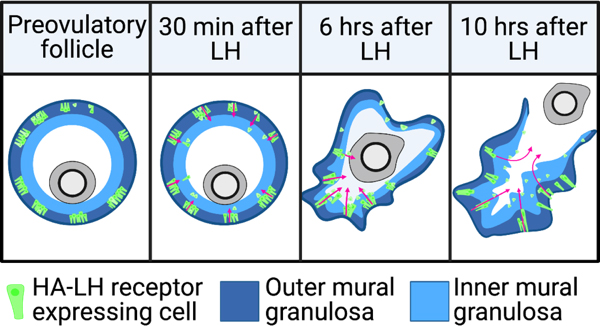
Luteinizing hormone stimulates ingression of granulosa cells within the mouse preovulatory follicle: a new component in the complex sequences of structural changes in the follicle that lead to ovulation. See Poster
For these studies, an endogenous LH surge was induced by injecting mice with kisspeptin (Owen et al., 2021). The peak of the LH surges occurs at ~1.5 hours after kisspeptin injection. By 2 hours after kisspeptin (30 minutes after the peak of the LH surge), LH receptor expressing cells began to migrate inwards into the follicle. Ovulation occurs at 11-12 hours after kisspeptin injection. Does LH-induced migration of the mural granulosa cells contribute to causing follicle rupture at ovulation? See Corie Owen's web page, and Owen and Jaffe, 2024.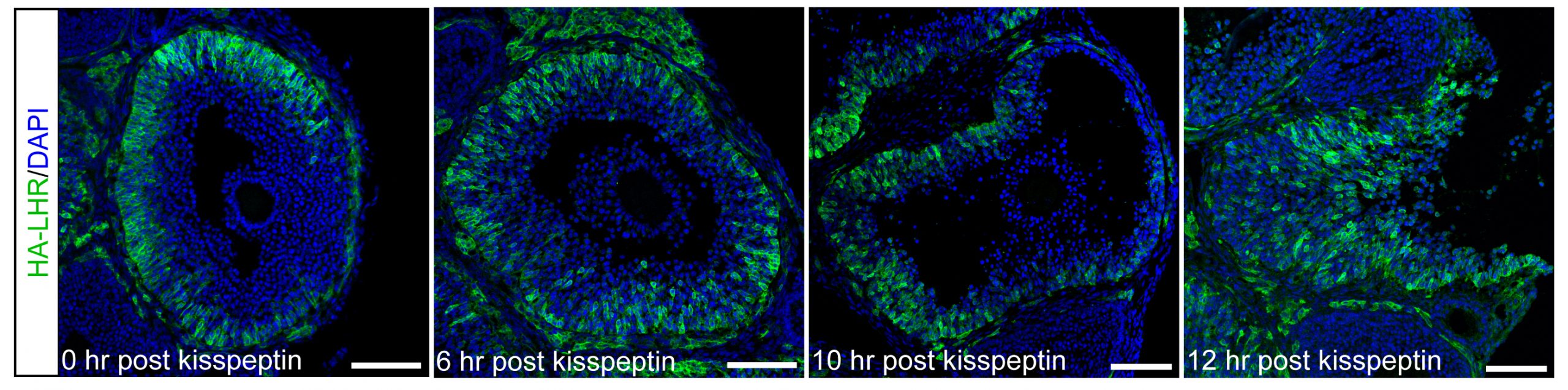
Movie: Microinjection of an antral follicle-enclosed mouse oocyte
Selected Publications
Owen, C.M., Jaffe, L.A. 2025. Luteinizing hormone-induced changes in the structure of mammalian preovulatory follicles. Curr Top Dev Biol.162:259-282.
Norris, R.P., Jaffe, L.A. 2024. Granulosa cells alone, without theca cells, can mediate LH-induced oocyte meiotic resumption. Endocrinology 165(3)bqad200.
Egbert, J.R., Silbern, I., Uliasz, T.F., Lowther, K.M., Yee, S.-P., Urlaub, H., Jaffe, L.A. 2024. Phosphatases modified by LH signaling in ovarian follicles: testing their role in regulating the NPR2 guanylyl cyclase. Biology of Reproduction 110:99-112.
Owen, C.M., Jaffe, L.A. 2024. Luteinizing hormone stimulates ingression of mural granulosa cells within the mouse preovulatory follicle. Biology of Reproduction 110:288-299.
Owen, C.M., Zhou, X., Bernard, D.J., and Jaffe, L.A. (2021). Kisspeptin-54 injection induces a physiological LH surge and ovulation in mice. Biol Reprod. 104:1181-1183.
Shuhaibar, L.C., Kaci, N., Egbert, J.R., Horville, T., Loisay, L., Vigone, G., Uliasz, T.F., Dambroise,E., Swingle, M.R., Honkanen, R.E., Duplan, M.B., Jaffe, L.A., Legeai-Mallet, L. (2021) Phosphatase inhibition by LB-100 enhances BMN-111 stimulation of bone growth..pdf JCI insight. 6 (9):141426.
Baena, V., Owen, C.M., Uliasz, T.F., Lowther, K.M., Yee, S.-P., Terasaski, M., Egbert, J.E., and Jaffe, L.A. (2020). Cellular heterogeneity of the LH receptor and its significance for cyclic GMP signaling in mouse preovulatory follicles. Endocrinology 161 (7):bqaa074.
Egbert, J.R., Yee, S.-P., and Jaffe, L.A. (2018). Luteinizing hormone signaling phosphorylates and activates the cyclic GMP phosphodiesterase PDE5 in mouse ovarian follicles, contributing an additional component to the hormonally induced decrease in cyclic GMP that reinitiates meiosis. Develop. Biol. 435:6-14.
Shuhaibar, L.C., Robinson, J.W., Vigone, G., Shuhaibar, N.P., Egbert, J.R., Baena, V., Uliasz, T.V., Kaback, D., Yee, S.-P., Feil, R., Fisher, M.C., Dealy, C.N., Potter, L.R., and Jaffe, LA. (2017). Dephosphorylation of the NPR2 guanylyl cyclase contributes to inhibition of bone growth by fibroblast growth factor. eLife 6:e31343.
Jaffe LA and Egbert JR, (2017) Regulation of Mammalian Oocyte Meiosis by Intercellular Communication Within the Ovarian Follicle. Annu. Rev. Physiol. 79: 237-260.
Egbert J.R., Uliasz T.F., Shuhaibar, L.C., Geerts A., Wunder F., Kleiman R.J., Humphrey J.M., Lampe P.D., Artemyev, N.O., Rybalkin, S.D., Beavo, J.A., Movsesian, M.A., and Jaffe, L.A. (2016) Luteinizing hormone causes phosphorylation and activation of the cyclic GMP phosphodiesterase PDE5 in rat ovarian follicles, contributing, together with PDE1 activity, to the resumption of meiosis. Biol. Reprod. 94(5):110. Supplement
Shuhaibar, L.C., Egbert, J.R., Edmund, A.B., Uliasz, T.F., Dickey, D.M., Yee, S.P., Potter, L.R., and Jaffe, L.A. (2016). Dephosphorylation of juxtamembrane serines and threonines of the NPR2 guanylyl cyclase is required for rapid resumption of oocyte meiosis in response to luteinizing hormone. Dev Biol. 409:194-201. Supplement
Shuhaibar, L.C., Egbert, J.R., Norris, R.P., Lampe, P.D., Nikolaev, V.O., Thunemann, M., Wen, L., Feil, R., and Jaffe, L.A. (2015). Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc. Natl. Acad. Sci. USA 112:5527-5532.
Egbert, J.R., Shuhaibar, L.C., Edmund, A.B., Van Helden, D.A., Robinson, J.W., Uliasz, T.F., Baena, V., Geerts, A., Wunder, F., Potter, L.R., and Jaffe, L.A. (2014). Dephosphorylation and inactivation of the NPR2 guanylyl cyclase in the granulosa cells contributes to the LH-induced decrease in cGMP that causes meiotic resumption in rat oocytes. Development. 141:3594-3604.
Norris, R.P., Ratzan, W.J., Freudzon, M., Mehlmann, L.M., Krall, J., Movsesian, M.A., Wang, H., Ke, H., Nikolaev, V.O., and Jaffe, L.A. (2009). Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136: 1869-1878. Supplement
Jaffe, L.A., Norris, R.P., Freudzon, M., Ratzan, W.J., and Mehlmann, L.M. (2009). Microinjection of follicle-enclosed mouse oocytes. Methods Mol. Biol. 518:157-173.
Jaffe, L.A., and Terasaki, M. 2004. Quantitative microinjection of oocytes, eggs and embryos. Meth. Cell Biol. 74: 219-242.
Mehlmann, L.M., Saeki, Y., Tanaka, S., Brennan, T.J., Evsikov, A.V., Pendola, F.L., Knowles, B.B., Eppig, J.J., and Jaffe, L.A. 2004. The Gs linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 306: 1947-1950. Supplement
Mehlmann, L.M., Jones,T.L.Z, and Jaffe, L.A. 2002. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science 297: 1343-1345.
Jaffe, L.A. 1993. The experimental process: the electrical polyspermy block. Essay in the textbook Molecular and Cellular Biology, by Stephen L. Wolfe. Wadsworth Publishing Co., pp. 1110-1111.
Jaffe, L.A. 1976. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature 261:68‑71.
Research in the Jaffe lab is supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R37HD014939).