 Associate Professor
Associate Professor
Department of Cell Biology
Contact
Phone: 860-679-2703
Email: lmehlman@uchc.edu
Office: E6052
UConn Health
263 Farmington Avenue
Farmington, CT 06030
Research Interests
Our lab is interested in elucidating cytoplasmic changes that occur in the mammalian oocyte that are important for the egg to become developmentally competent, as well as the mechanisms that cause these cytoplasmic changes to occur. A complete understanding of these events will contribute to improving methods for maturing oocytes in vitro.
Mammalian oocytes are stored within ovarian follicles at the prophase stage of meiosis. They remain in prophase I as they and their associated follicles grow, and until a preovulatory surge of luteinizing hormone (LH) from the pituitary stimulates meiotic resumption to the metaphase II stage. During the period of oocyte growth, meiotic arrest is maintained due to an insufficient amount of maturation-inducing proteins, in particular the cell cycle kinase CDK1. However, as CDK1 accumulates in the oocyte, the oocyte becomes competent to mature and meiotic arrest shifts to a dependency on cAMP. Work in my lab has established that the oocyte produces cAMP by the constitutive activity of the Gs-coupled receptor, GPR3 (Mehlmann et al., 2004). GPR3 localizes in the plasma membrane and throughout the oocyte in early endosomes, and although it is constitutively active, its activity appears to be regulated via endocytosis (Lowther et al., 2011, 2013). Oocytes lacking GPR3 are unable to maintain meiotic arrest and resume meiosis spontaneously in the follicle, in the absence of an LH surge. At the same time that the oocyte is producing cAMP, cAMP hydrolysis is prevented in the oocyte due to the inhibition of the phosphodiesterase, PDE3A, by follicle-derived cGMP (Norris et al., 2009). High cGMP enters the oocyte through gap junctions and ensures that cAMP levels remain high until LH stimulates the follicle cells to reduce cGMP both in the follicle as well as the oocyte. This in turn activates PDE3A in the oocyte to hydrolyze cAMP and cause meiotic resumption. Available evidence indicates that similar mechanisms also function in human oocytes (DiLuigi et al., 2008).
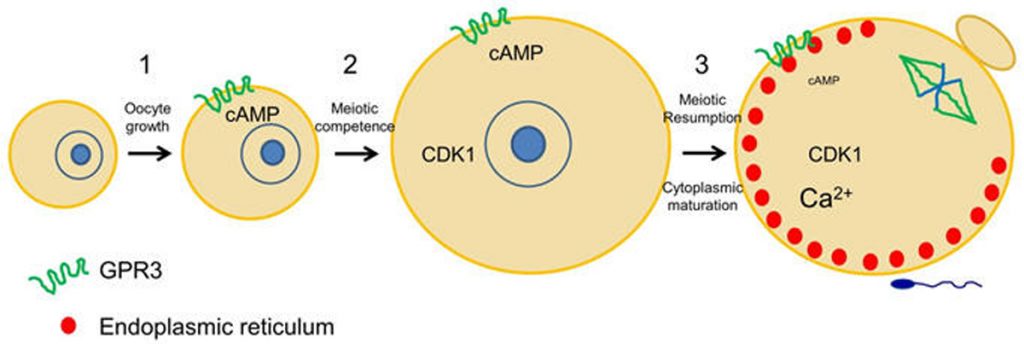
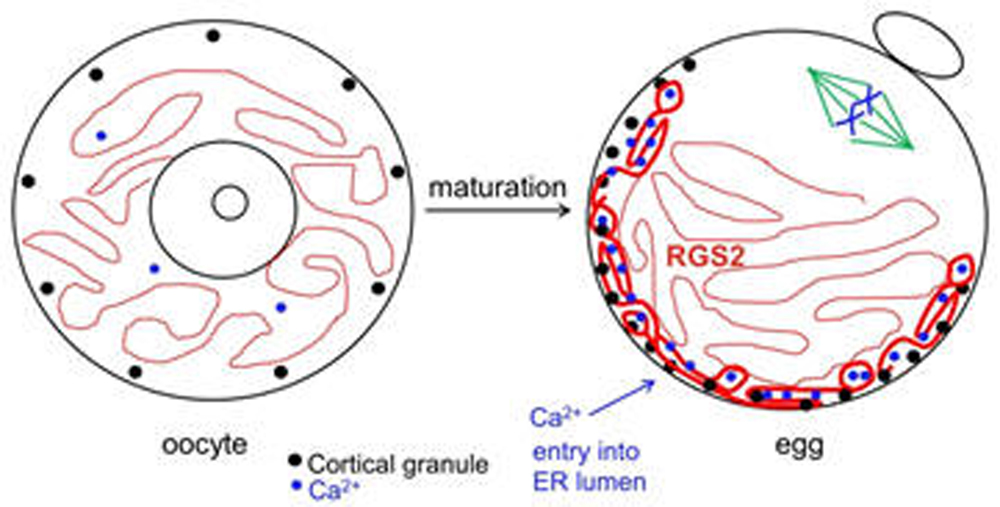
Once LH stimulates the oocyte to mature, it undergoes numerous cytoplasmic changes during its transition to a fertilizable egg. Many of these changes are not well understood. However, it is well established that during maturation, the oocyte’s endoplasmic reticulum (ER) undergoes a dramatic reorganization, such that the ER accumulates in the egg cortex (Mehlmann et al., 2005; Mann et al., 2010). As the primary Ca2+ storage organelle, the ER thereby becomes situated in the immediate region of the fertilizing sperm, which triggers Ca2+ release through an IP3-mediated signaling cascade. This release of Ca2+ is critical for successful development of the newly-formed embryo. Other changes that occur during oocyte maturation are an influx of Ca2+ into the ER lumen, as well as a rearrangement of cortical granules to the cortex opposite the meiotic spindle. In addition, the protein RGS2 becomes expressed during oocyte maturation to inhibit premature Ca2+ release prior to fertilization (Bernhardt et al., 2015). The ability to release cortical granules at fertilization also develops during oocyte maturation. Cortical granule exocytosis is important for preventing polyspermy following fertilization. Our lab has been investigating mechanisms by which mature eggs exocytose cortical granules after fertilization.
Our lab is interested in elucidating cytoplasmic changes that occur in the oocyte that are important for the egg to become developmentally competent, as well as the mechanisms that cause these cytoplasmic changes to occur. A complete understanding of these events will contribute to improving methods for maturing oocytes in vitro.
Recent data:
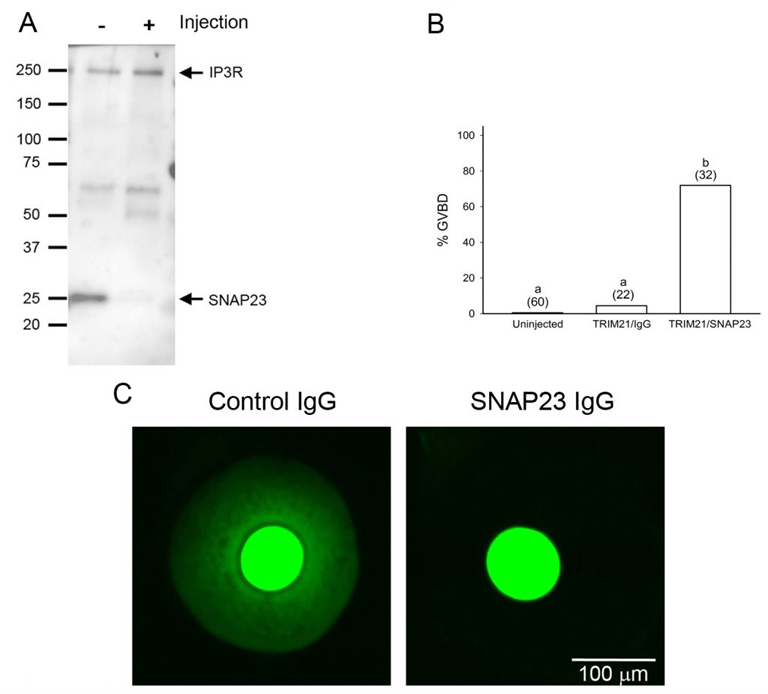
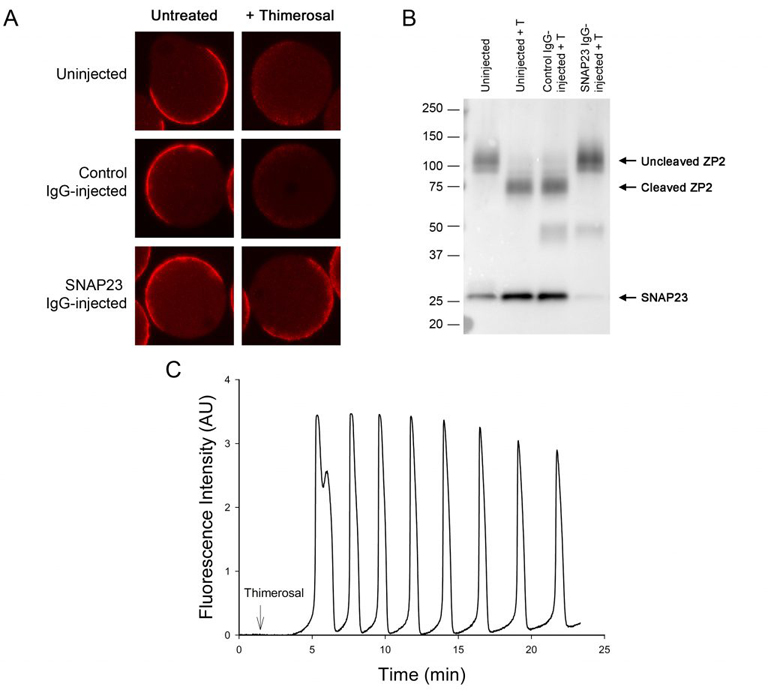
Oocytes normally undergo the processes of endocytosis and exocytosis. In the immature oocyte, endocytosis appears to be associated with the regulation of meiosis, as preventing endocytosis causes the buildup of cAMP in the oocyte and blocks spontaneous meiotic resumption in oocytes that were isolated from follicles (Lowther et al., 2011). Using a newly developed method called Trim-away, we have shown that depleting the exocytotic SNARE protein, SNAP23, in follicle-enclosed oocytes causes them to undergo spontaneous maturation in the absence of LH (Mehlmann et al., 2019). This is attributed to the disconnection of the oocyte and the surrounding follicle cells, possibly due to the inability of the oocyte to place connexin 37 in the oocyte’s plasma membrane. SNAP23 is also required for cortical granule exocytosis in the mature egg following fertilization.
New research on reproductive function in transgender males following testosterone therapy:
Recently, we have become interested in the quality of eggs retrieved from female mice that have been treated with testosterone. The rationale for the study is that transgender males (biologically female) often take high levels of testosterone to achieve desired male secondary sex characteristics such as lower voice and hair growth. Many transgender males wish to have biological children, but the effects of testosterone therapy on their eventual fertility is largely unknown. Current standard of care for transgender men who want to undergo egg retrieval is to first stop testosterone treatments and allow menses to resume; however, this treatment often triggers gender dysphoria in men and might cause them to avoid treatment, despite their desire for biological children.
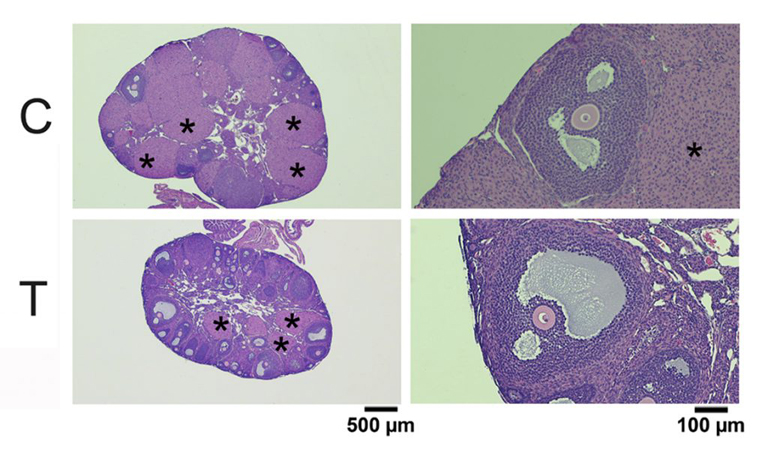
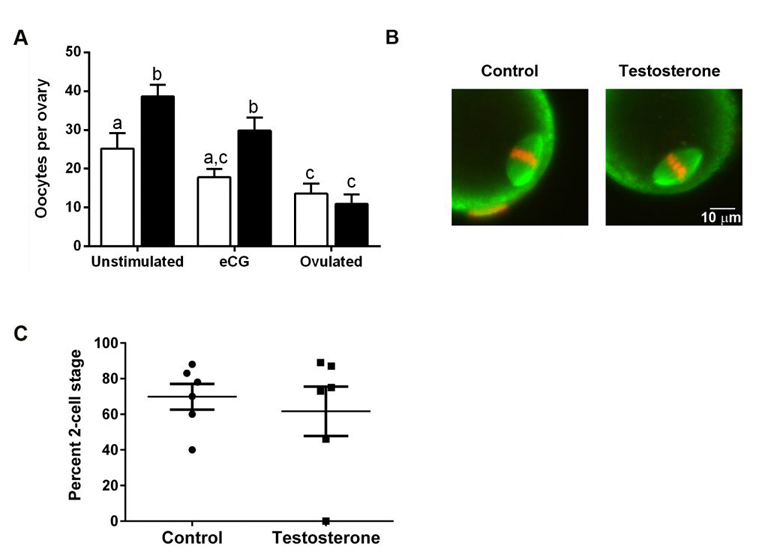
To test whether testosterone therapy needs to stop prior to obtaining eggs, we injected female mice with testosterone for 6 weeks. This treatment raised serum testosterone levels and had masculinizing effects such as clitoromegaly and caused the estrous cycle to stop. Ovaries from testosterone-treated mice weighed less than control ovaries but were histologically normal. Oocytes retrieved from testosterone-treated mice were meiotically competent and developed normal meiotic spindles after in vitro maturation. Importantly, testosterone-treated mice ovulated similar numbers of fertilizable eggs as controls in response to gonadotropin treatment. These experiments provide promising data that it might not be necessary for transgender men to be taken off of testosterone prior to gonadotropin treatment as the normal course of egg retrieval. Full results may be obtained using the following link: https://www.biorxiv.org/content/10.1101/2020.04.09.033803v1. More work is being done to examine successful development of offspring following fertilization of eggs retrieved from testosterone-treated mice.
Selected Publications
Lisa M. Mehlmann, Tracy F. Uliasz, Siu-Pok Yee, Deborah Kaback, Katie M. Lowther. (2024) Generation and Characterization of a TRIM21 Overexpressing Mouse Line. Genesis; 62:e23616. https://doi.org/10.1002/dvg.23616.
Prachi Godiwala, Tracy F. Uliasz, Katie M. Lowther, Deborah Kaback, and Lisa M. Mehlmann. (2023) Puberty Suppression Followed by Testosterone Therapy Does Not Impair Reproductive Potential in Female Mice. Endocrinology, 164, 1–10.
Bartels CB, Uliasz TF, Lestz L, Mehlmann LM. (2021) Short term testosterone use in female mice does not impair fertilizability of eggs: Implications for the fertility care of transgender males. Hum Reprod., 36: 189-198.
Eisa AA, Bang S, Crawford KJ, Murphy EM, Feng WW, Dey S, Wells W, Kon N, Gu W, Mehlmann LM, Viyayaraghavan S, Kurokawa M.(2020) X-Linked Huwe1 Is Essential for Oocyte Maturation and Preimplantation Embryo Development. iScience., 23 (9):101523.
Mehlmann, LM, Uliasz, TF, Lowther, KM. (2019) SNAP23 is required for constitutive and regulated exocytosis in mouse oocytes. Biol of Reprod., 101:338–346.
Firmani LD, Uliasz TF, Mehlmann LM. 2018. The switch from cAMP-independent to cAMP-dependent arrest of meiotic prophase is associated with coordinated GPR3 and CDK1 expression in mouse oocytes. Dev Biol. 434:196-205.
Bernhardt, M.L., Lowther, K.M., Padilla-Banks, E., McDonough, C.E., Lee, K.N., Evsikov, A.V., Uliasz, T.F., Chidiac, P., Williams, C.J., and
Mehlmann, L.M. 2015. Regulator of G-protein signaling 2 (RGS2) suppresses premature calcium release in mouse eggs. Development 142: 2633-2640.
Lowther, K.M. and Mehlmann, L.M. 2015. Embryonic Poly(A)-Binding Protein Is Required During Early Stages of Mouse Oocyte Development for Chromatin Organization, Transcriptional Silencing, and Meiotic
Competence. Biol Reprod. 93:43.
Mehlmann, L.M. 2013. Signalling for meiotic resumption in granulosa cells, cumulus cells, and oocyte. In: Mammalian Oogenesis, Coticchio, De Santis, and Albertini, eds. Springer Publishing Company, New York, NY, pp. 171-182.
Lowther, K.M., Uliasz, T.F., Gotz, K.R., Nikolaev, V.O., and Mehlmann, L.M. 2013. Regulation of constitutive GPR3 signaling and surface localization by GRK2 and b-arrestin-2 overexpression in HEK293 cells. PLoS One 8: e65365.
Mehlmann, L.M. 2013. Losing mom’s message: requirement for DCP1A and DCP2 in the degradation of maternal transcripts during oocyte maturation. Biol Reprod. 88: 1-2.
Guzeloglu-Kayisli, O., Lalioti, M.D., Aydiner, F., Sasson, I., Ilbay, O., Sakkas, D., Lowther, K.M., Mehlmann, L.M, and Seli, E. 2012. Embryonic poly(A)-binding protein (EPAB) is required for oocyte maturation and female fertility in mice. Biochem J. 446:47-58.
Lowther, K.M., Nikolaev, V.O., and Mehlmann, L.M. 2011. Endocytosis in the mouse oocyte and its contribution to cAMP signaling during meiotic arrest. Reproduction 141:737-747.
Mann, J.S., Lowther, K.M., and Mehlmann, L.M. 2010. Reorganization of the endoplasmic reticulum and development of Ca2+ release mechanisms during meiotic maturation of human oocytes. Biol. Reprod. 83:578–583.
Lowther, K.M., Weitzman, V.N., Maier, D., and Mehlmann, L.M. 2009. Maturation, fertilization, and the structure and function of the endoplasmic reticulum in cryopreserved mouse oocytes. Biol. Reprod. 81:147-154.
Norris, R.P., Ratzan, W.J., Freudzon, M., Mehlmann, L.M., Krall, J., Movsesian, M.A., Wang, H., Ke, H., Nikolaev, V.O., and Jaffe, L.A. 2009. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136:1869-1878.
DiLuigi, A., Weitzman, V.N., Pace, M.C., Siano, L.J., Maier, D., and Mehlmann, L.M. 2008. Meiotic arrest in human oocytes is maintained by a Gs signaling pathway. Biol. Reprod. 78:667-672.
Vaccari, S., Horner, K., Mehlmann, L.M., and Conti, M. 2008. Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev. Biol. 316:124-134.
Norris, R.P., Freudzon, M., Mehlmann, L.M., Cowan, A.E., Simon, A.M., Paul, D.L., Lampe, P.D., and Jaffe, L.A. 2008. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135:3229-3238.
Norris, R.P., Freudzon, L., Freudzon, M., Hand, A.R., Mehlmann, L.M., and Jaffe, L.A. 2007. A Gs-linked receptor maintains meiotic arrest in mouse oocytes, but luteinizing hormone does not cause meiotic resumption by terminating receptor-Gs signaling. Dev. Biol. 310:240-249.
Mehlmann, L.M., Kalinowski, R.R., Ross, L.F., Parlow, A.F., Hewlett, E.L. and Jaffe, L.A. 2006. Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev. Biol. 299:345-355.
Mehlmann, L.M. 2005. Stops and Starts in Mammalian Oocytes: Recent Advances in Understanding the Regulation of Meiotic Arrest and Oocyte Maturation. Reproduction 130:791-798.
Freudzon, L., Norris, R.P., Hand, A.R., Tanaka, S., Saeki, Y., Jones, T.L.Z., Rasenick, M.M., Berlot, C.H., Mehlmann, L.M., and Jaffe, L.A. 2005. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J. Cell Biol. 171:255-265.
Mehlmann, L.M. 2005. Oocyte-specific expression of GPR3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev. Biol. 288:397-404.
Mehlmann, L.M., and Jaffe, L.A. 2005. SH2 domain-mediated activation of a SRC family kinase is not required to initiate Ca2+ release at fertilization in mouse eggs. Reproduction: 129: 557-564.
Mehlmann, L.M., Saeki, Y., Tanaka, S., Brennan, T.J., Evsikov, A.V. Pendola, F.L., Knowles, B.B., Eppig, J.J., and Jaffe, L.A. 2004. The G s -linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science: 306: 1947 – 1950.
Kalinowski, R.R., Berlot, C.H., Jones, T.L.Z., Ross, L.F., Jaffe, L.A., and Mehlmann, L.M. 2004. Maintenance of meiotic prophase arrest in vertebrate oocytes by a G s protein-mediated pathway. Dev. Biol. 267: 1-13.
Mehlmann, L.M., Jones, T.L.Z., and Jaffe, L.A. 2002. Meiotic arrest in the mouse follicle maintained by a G s protein in the oocyte. Science 297:1343-1345.
Runft, L.L., Jaffe, L.A., and Mehlmann, L.M. 2002. Egg activation at fertilization: Where it all begins. Dev. Biol. 245:237-254.