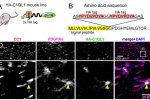
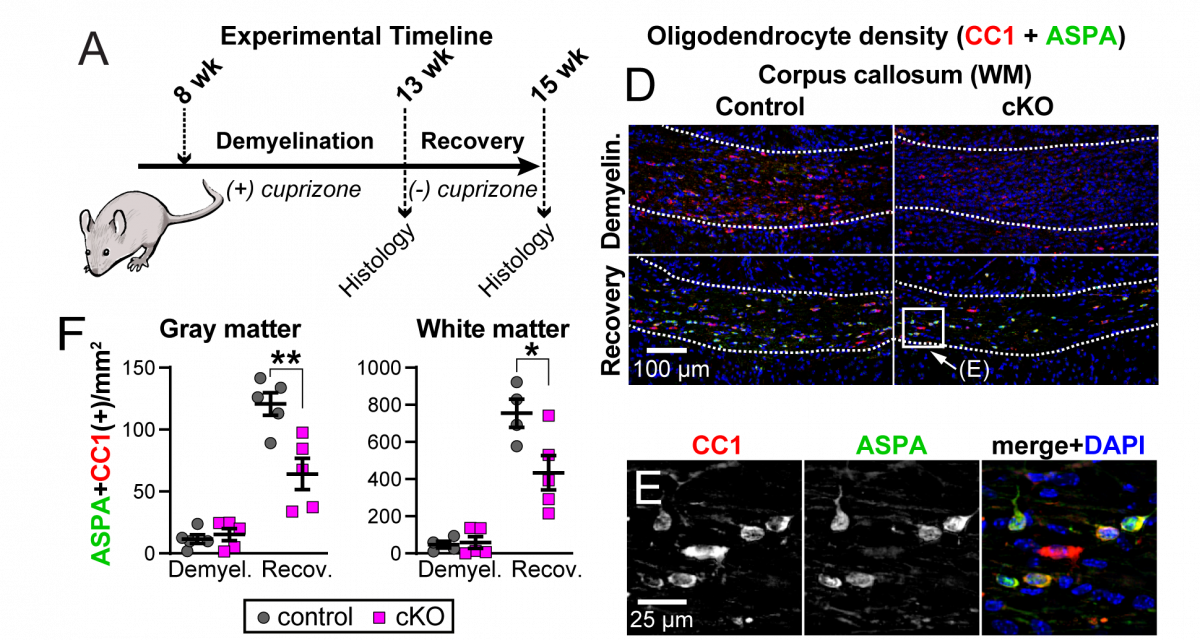
 The drug cuprizone when fed to mice causes demyelination and a death of oligodendrocytes (marked by co-expression of CC1 and ASPA). When returned to a normal diet, spontaneous recovery occurs. We found that mice lacking the C1ql1 gene do not recover as well, suggesting that the C1QL1 protein is beneficial for recovery from demyelination.
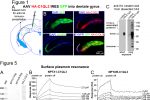
The drug cuprizone when fed to mice causes demyelination and a death of oligodendrocytes (marked by co-expression of CC1 and ASPA). When returned to a normal diet, spontaneous recovery occurs. We found that mice lacking the C1ql1 gene do not recover as well, suggesting that the C1QL1 protein is beneficial for recovery from demyelination.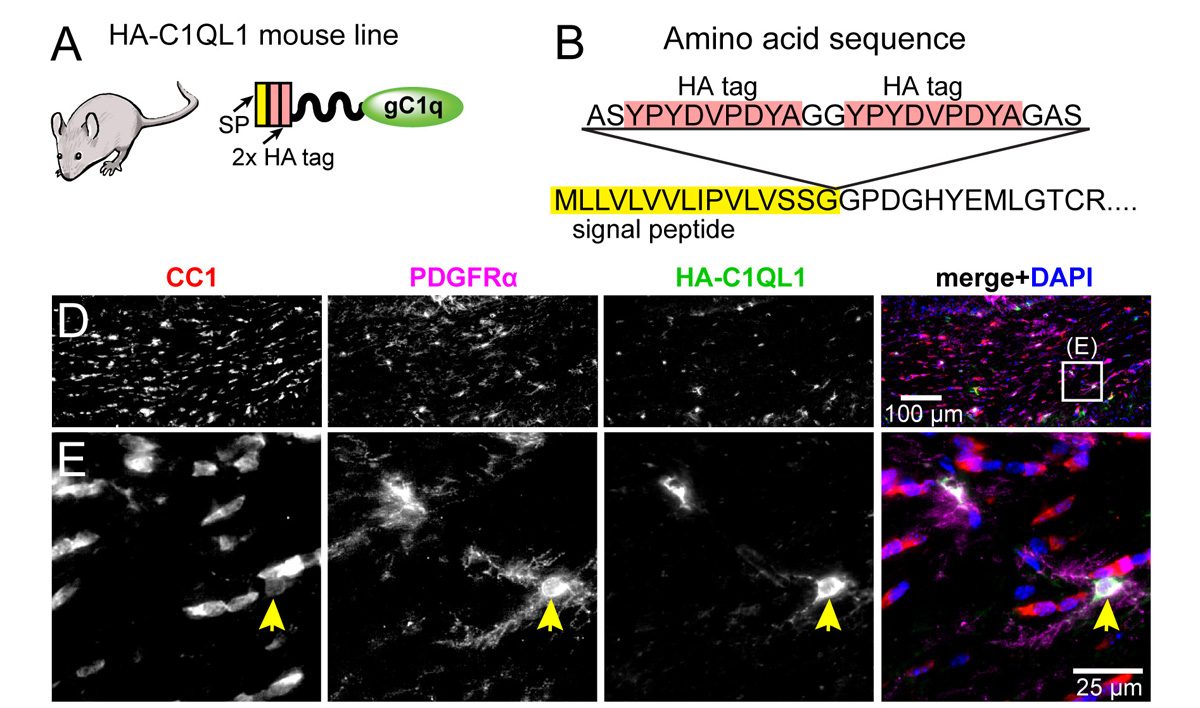 We have created novel HA epitope-tagged knock in mouse lines to facilitate biochemistry, map expression locations, and determine subcellular localization. We have discovered that C1QL1, previously assumed to be a synaptic protein expressed by neurons, is expressed in a glia sub-type called oligodendrocyte progenitor cells (OPCs; marked by the protein PDGFRα).
We have created novel HA epitope-tagged knock in mouse lines to facilitate biochemistry, map expression locations, and determine subcellular localization. We have discovered that C1QL1, previously assumed to be a synaptic protein expressed by neurons, is expressed in a glia sub-type called oligodendrocyte progenitor cells (OPCs; marked by the protein PDGFRα).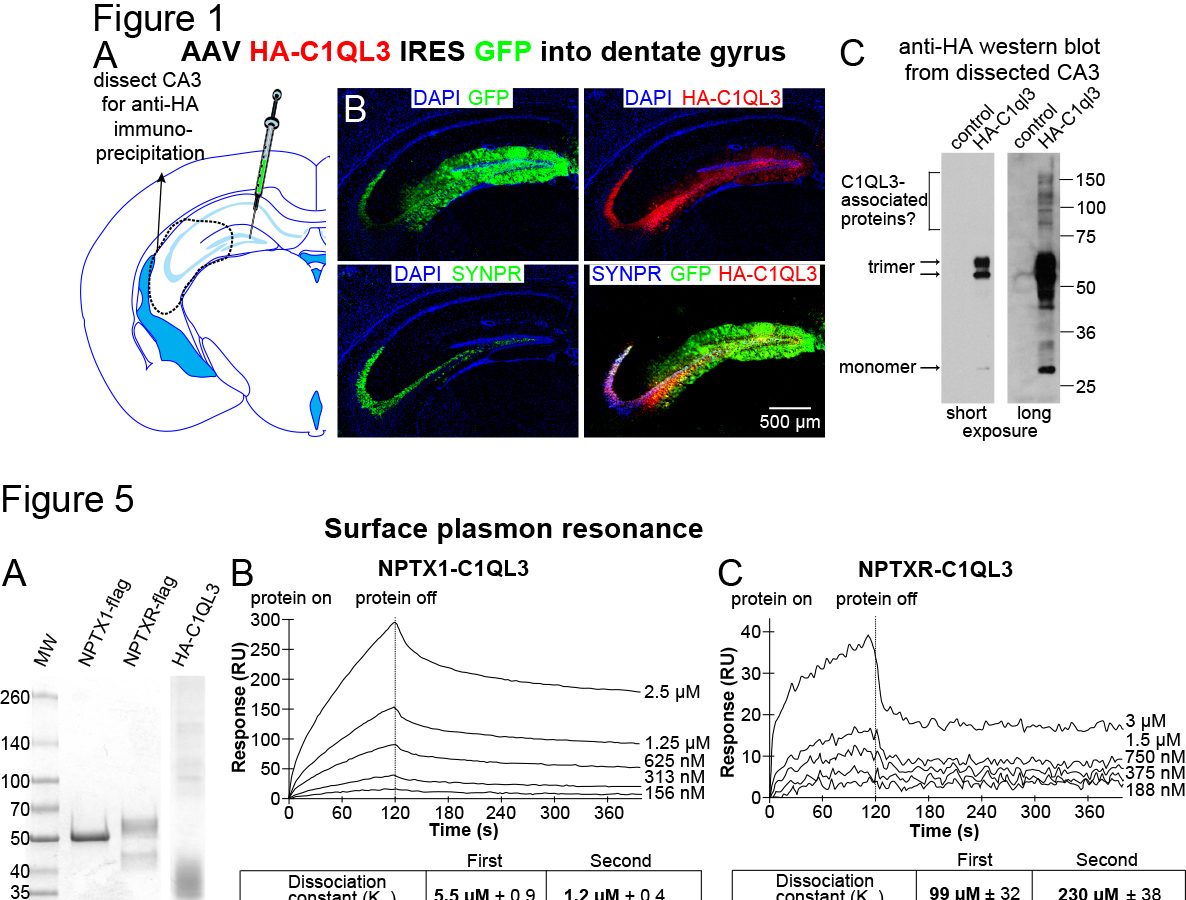 In our recent publication in the FASEB journal, we conducted a screen to identify novel C1QL3 binding partners, which revealed members of the neuronal pentraxin family (NPTX1 and NPTXR). The conclusion was confirmed using quantitative biochemical methods (surface plasmon resonance) to measure the binding affinity. We predict that the neuronal pentraxins are a component in a C1QL-mediated trans-synaptic adhesion complex.
In our recent publication in the FASEB journal, we conducted a screen to identify novel C1QL3 binding partners, which revealed members of the neuronal pentraxin family (NPTX1 and NPTXR). The conclusion was confirmed using quantitative biochemical methods (surface plasmon resonance) to measure the binding affinity. We predict that the neuronal pentraxins are a component in a C1QL-mediated trans-synaptic adhesion complex.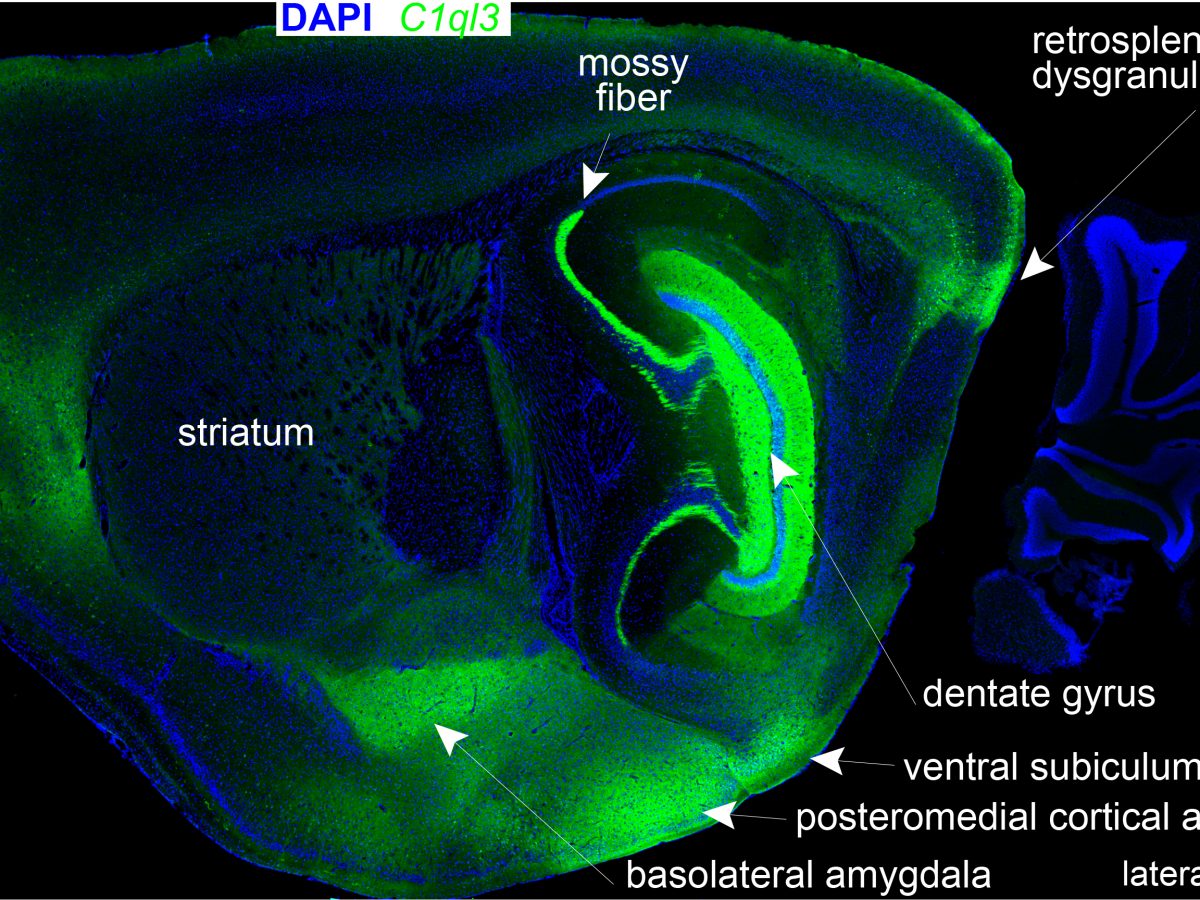 C1ql3 has a beautiful gene expression pattern. Any neuron expressing C1ql3 is marked by expression of an mVenus reporter ...(more)
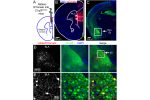
C1ql3 has a beautiful gene expression pattern. Any neuron expressing C1ql3 is marked by expression of an mVenus reporter ...(more)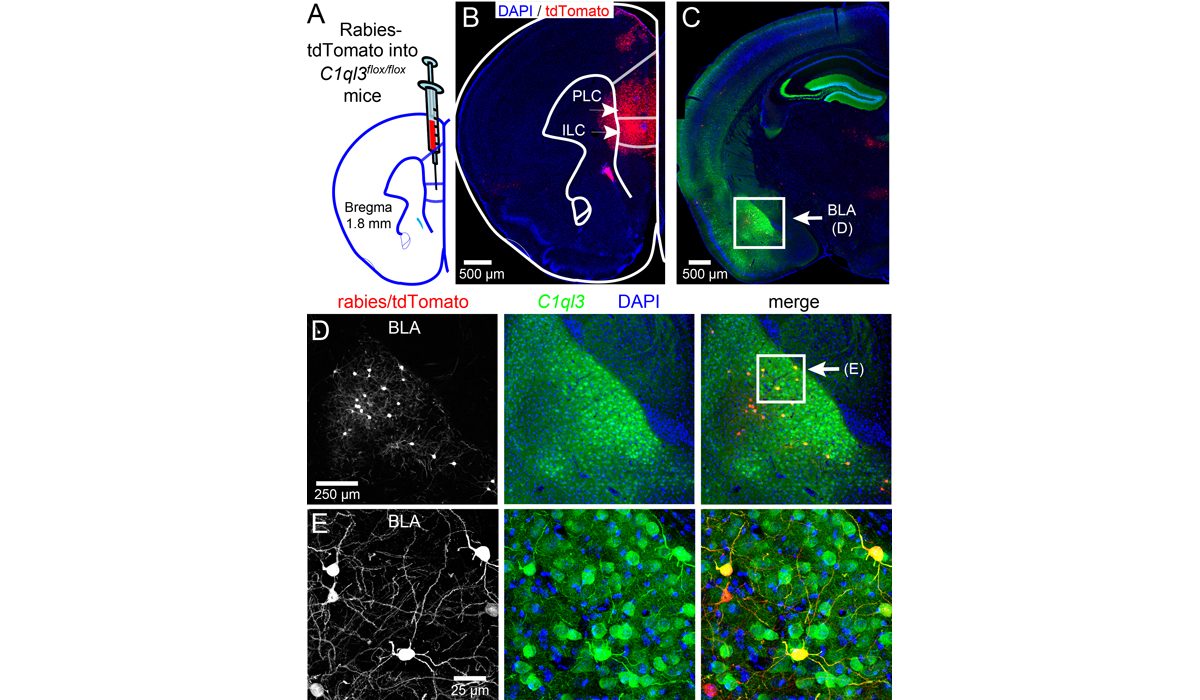 We discovered that C1ql3-expressing neurons in the basolateral amygdala (BLA) send axonal projections to the medial prefrontal cortex (mPFC). A rabies virus ...(more)
We discovered that C1ql3-expressing neurons in the basolateral amygdala (BLA) send axonal projections to the medial prefrontal cortex (mPFC). A rabies virus ...(more) The Martinelli lab performs numerous mouse behavior assays. Shown here are cocaine conditioned place preference and lithium conditioned ...(more)
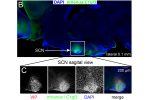
The Martinelli lab performs numerous mouse behavior assays. Shown here are cocaine conditioned place preference and lithium conditioned ...(more)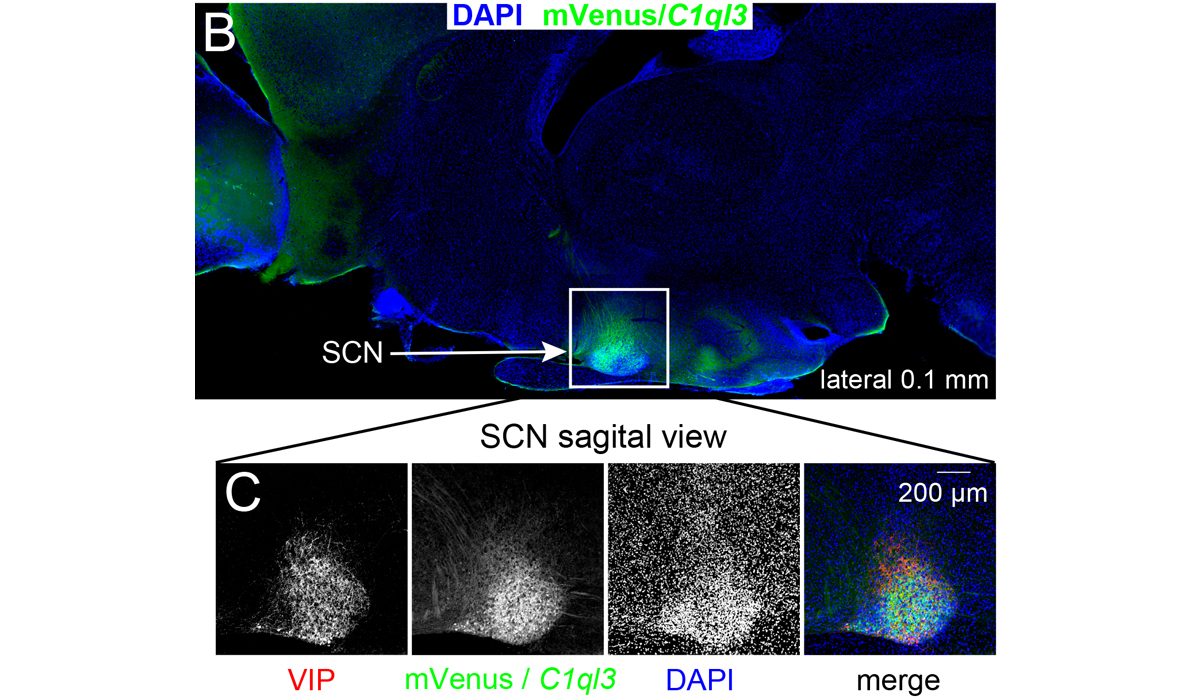 We surprisingly discovered that the C1ql3 gene was expressed in the suprachiasmatic nucleus ...(more)
We surprisingly discovered that the C1ql3 gene was expressed in the suprachiasmatic nucleus ...(more)
Contact Us
David C. Martinelli, Ph.D.
Assistant Professor of Neuroscience
Academic Office Location:
Neuroscience Department
UConn Health
263 Farmington Avenue
Farmington, CT 06030-3401
Phone: 860-679-2271
Lab Phone: 860-679-4989
Email: davidmartinelli@uchc.edu
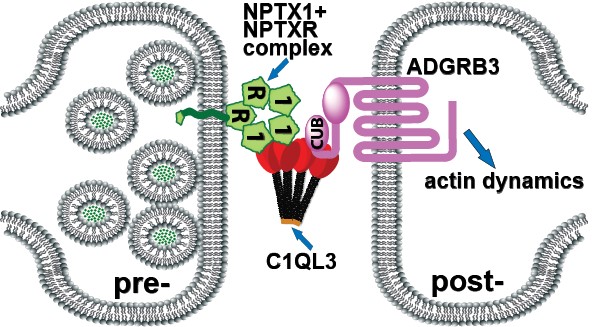
C1QL Binding
Project #1
A synapse is the fundamental structural unit by which neurons communicate. The importance of synapses cannot be overstated as their collective activities impact every moment of our subconscious and conscious mind. Furthermore, synapses are formed and continuously restructured throughout life based on different experiences, which ultimately shapes us as individuals with unique memories, thoughts, skills, and personalities. How the trillions of synapses in a brain are wired into functional neural networks and the mechanisms of experience-dependent synaptic plasticity are important outstanding questions.
We are particularly fascinated by synaptic adhesion proteins, which bind across the synaptic cleft to form a molecular interface between pre- and post-synaptic membranes and also initiate intercellular trans-synaptic signaling. These proteins are at the junction of our genes and experiences and are critical for initiating and stabilizing synaptic changes in a multitude of ways. Our research goal is to understand the molecular logic of how synaptic adhesion proteins orchestrate synaptic formation, modification, and function, and to ultimately provide an explanation for how these events influence behaviors, in particular the aberrant behaviors associated with neuropsychiatric diseases.
The immediate focus of our research is on the neuronally secreted C1q-like family of proteins. The lab studies the biochemical interactions with their pre- and post-synaptic protein binding partners, their synaptic signaling activities, and their eventual behavioral consequences. A priori, their localization in the synaptic cleft almost predestines them to have neuropsychiatric disease relevance. Genetic analyses of C1q-like mutant mice revealed behavioral abnormalities potentially resembling several neuropsychiatric diseases, including ADHD and addiction predisposition. How C1q-like proteins and their binding partners influence synapses and ultimately behaviors is unknown. The techniques used in the lab to answer these questions encompass biochemistry, genetics, cell biology, circuit analysis using viral vectors, and behavioral assays.
C1Q-like proteins are released pre-synaptically from excitatory neurons into the synaptic cleft, where they interact with a post-synaptically localized G protein-coupled receptor (GPCR) called ADGRB3 (a.k.a. BAI3). We are currently investigating the hypothesis that C1QL3 binds to proteins known as neuronal pentraxins on the pre-synaptic side to complete a trans-synaptic adhesion complex, and that this complex will both promote synapse formation/maintenance and regulate behaviors dependent on C1QL3-positive circuits.
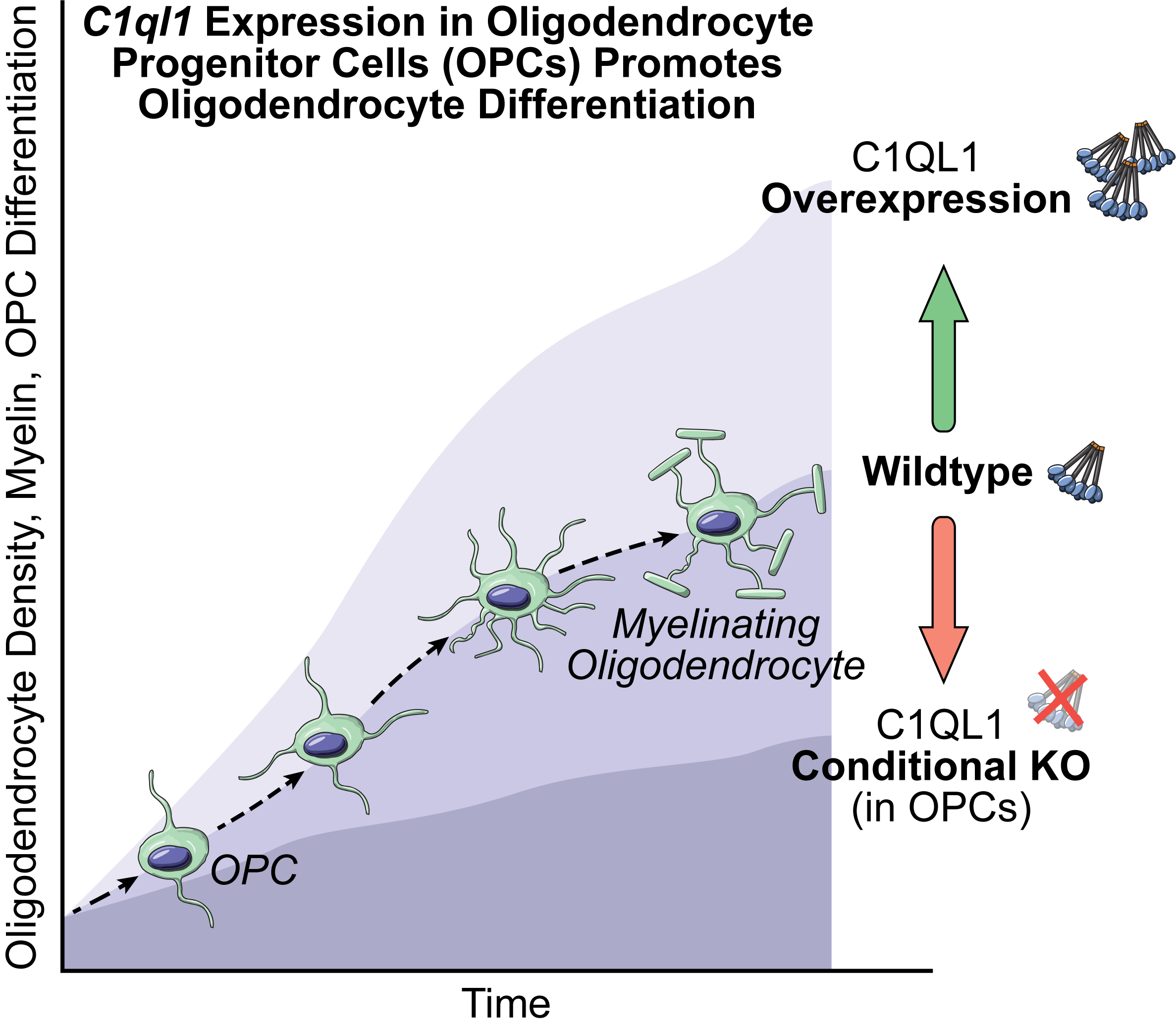
C1ql1 expression in OPCs
Project #2
In multiple sclerosis, the protective myelin sheath around neurons in the brain degenerates. This protective sheath is provided by specialized cells called oligodendrocytes. The healthy brain normally has a mechanism in place to produce new oligodendrocytes that can replace the protective sheathes if they are lost or damaged. To do so, the brain maintains a population of cells known as oligodendrocyte progenitor cells (OPCs). The brain has the ability to transform some of these OPCs into mature oligodendrocytes, which can then replace any lost myelin. How OPCs are instructed to mature into oligodendrocytes is poorly understood, but it is clear that this process is restricted or lost during multiple sclerosis disease progression, which includes progressively increasing symptoms and disability. This project endeavors to identify a previously unknown pathway by which the brain can use to create new oligodendrocytes from its resident pool of OPCs, thus promoting myelination after demyelinating injury.
We have discovered that a protein (C1QL1) not previously associated with oligodendrocytes is made by OPCs, and we hypothesize that it initiates a signaling pathway leading to OPC maturation into oligodendrocytes. To test this both in vivo and in vitro, we are using multiple genetically modified mouse lines and are creating novel viruses for various molecular genetic manipulations.
Results from this study will contribute important new information on the science underlying the maturation of oligodendrocytes required for brain repair in patients with multiple sclerosis. Our studies will interrogate the function of this protein to promote the production of new oligodendrocytes, which could then be applied to the development of small molecule drug(s) designed to mimic the activity of this protein to promote brain repair.