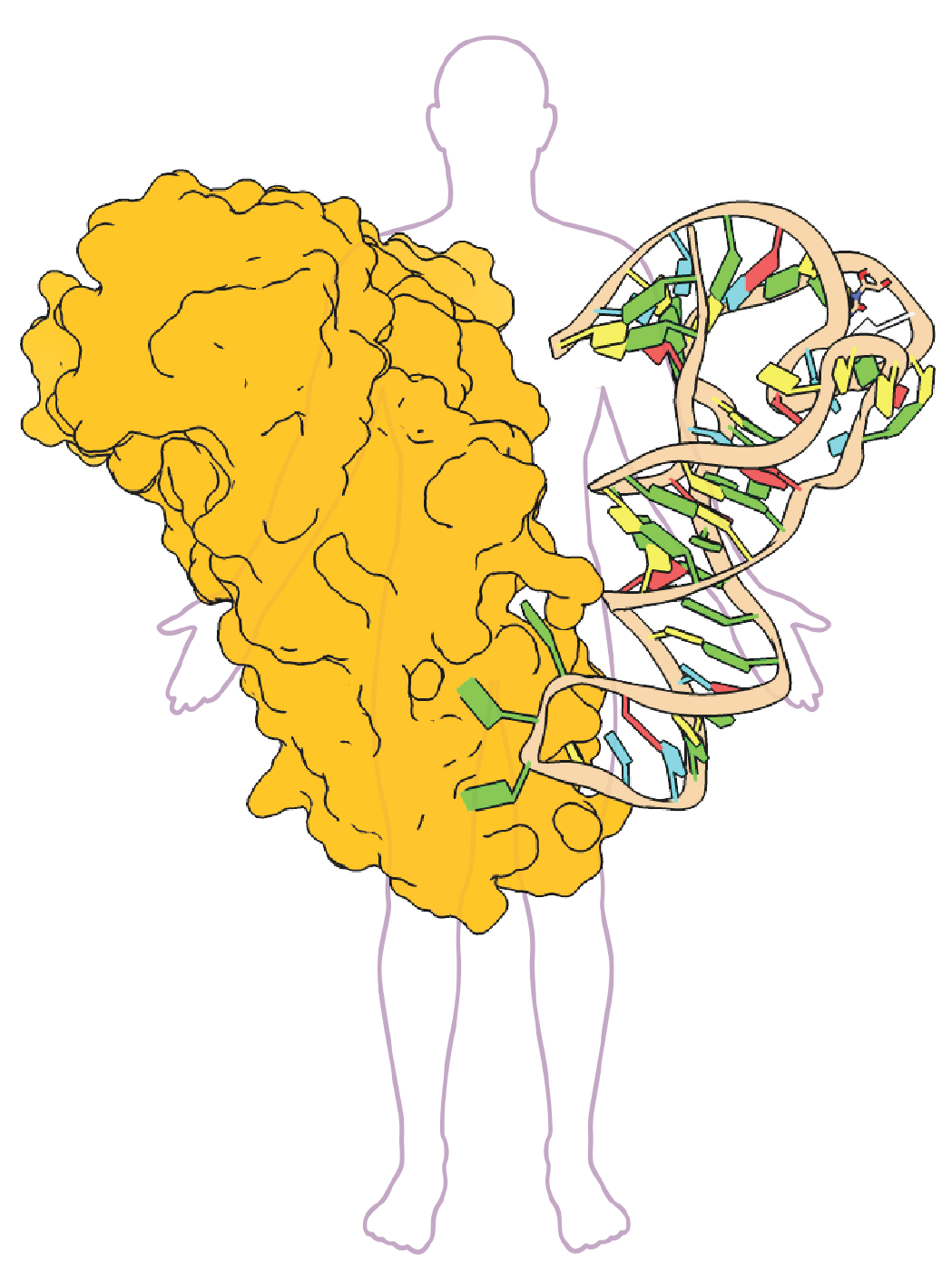
Adaptative mechanisms of translation in microbial pathogens
We study the molecular bases that enable pathogenic bacteria to endure, acclimate, and prevail in the unwelcoming conditions of their host. Our research seeks to understand how these microbes regulate protein synthesis (translation) in response to interactions with their host, especially during colonization and infection.
We focus on the function of a family of translation factors known as aminoacyl-tRNA synthetases (aaRS). These enzymes are indispensable for life as they are responsible for the accurate and efficient translation of the genetic code during gene expression. Our aim is to understand the biological role of aaRSs during host-pathogen interactions as potential infection factors and druggable targets using a combination of biochemical, biophysical, molecular, cellular, multi-omics, and computational approaches.
tRNA-based medicines for treatment of nonsense mutations
Nonsense mutations that introduce premature termination codons (PTCs) in the protein-coding regions of genes cause ~10% of human genetic diseases. PTCs prematurely terminate gene translation and produce truncated, non-functional proteins. Unfortunately, very few treatment options are available for patients suffering from PTC-related conditions.
Efforts in our lab aim to design, engineer, and develop suppressor tRNAs (sup-tRNAs) that translate disease-causing PTCs, restoring protein synthesis from mutated genes. Sup-tRNAs offer an exciting therapeutic avenue to treat diseases caused by PTCs.
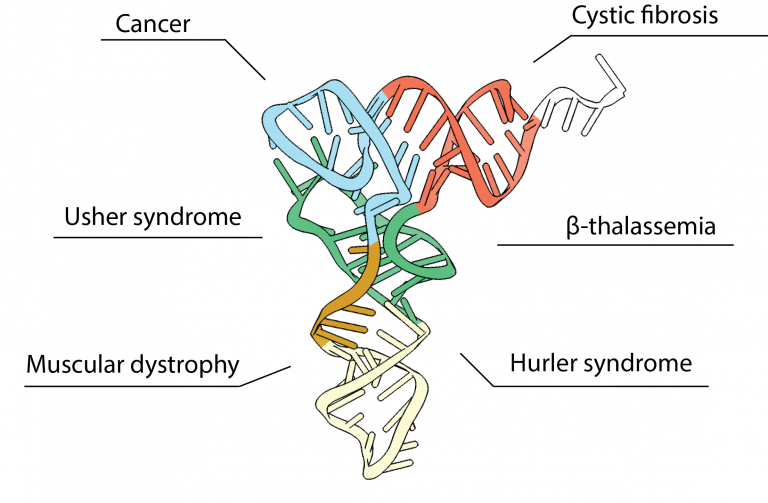
Human pathogenic mutations in aminoacyl-tRNA synthetases
Due to their vital role in protein synthesis, mutations in human aminoacyl-tRNA synthetases (aaRSs) can cause untreatable diseases. Our lab investigates the functional consequences and molecular mechanisms associated with aaRS mutations using molecular biology, biochemical and biophysical methods. Our research aims to provide knowledge to facilitate the design and development of therapies to treat aaRS-related diseases.