Research
The Hurley Lab examines molecular mechanisms by which members of the fibroblast growth factor (FGFs) and fibroblast growth factor receptor (FGFR) families, regulate, bone remodeling and disorders of bone, skeletal muscle/bone crosstalk and joint homeostasis. Specifically, FGF2 global and conditional knockout and FGF2 isoform transgenic mice are utilized in loss and gain of function experiments to elucidate the role of FGF2 in disorders of bone including osteoporosis, fracture healing, osteoarthritis as well skeletal muscle aging.
Our studies have shown that FGF2 expression in osteoblasts is highly regulated by hormones, growth factors and cytokines including parathyroid hormone, transforming growth factor beta, interleukin-1, prostaglandins and bone morphogenetic protein-2. The role of FGF2 in modulating their responses in bone is under investigation
High molecular weight FGF2 isoforms regulate the phosphatonin FGF23, and is increased in the Hyp mouse model of the rare disorder X-linked hypophosphatemic rickets. We developed novel FGF2 isoform transgenic mice (HMWFGFTg) that has led to a new area of focus to understand the role of nuclear localized FGF2 isoforms in chondrodysplasias, rickets and osteomalacia due to phosphate wasting, and osteoarthropathy. Studies to delineating the epigenetic mechanism by which the nuclear localized high molecular weight isoforms of FGF2 mediate growth-plate abnormalities and osteoarthropathy are ongoing. HMWFGF2Tg mice also demonstrate mandibular and dental pulp abnormalities that are under investigation.
Differential effects of FGF2 isoform knockout on bone and joint homeostasis in mice are also in progress.
Current Collaborations
The Hurley Lab is part of a collaborative group of scientists and clinician-scientists in the School of Medicine focusing on the biology and therapeutic targeting of musculoskeletal disorders. In addition, Dr. Hurley has established special collaborations with faculty in the School of Dental Medicine and the School of Engineering directed toward dissecting the molecular mechanisms of FGF ligands and receptors in bone regeneration and osteoarthritis.
Research Opportunities
If you are interested in learning about current ongoing projects, please contact Dr. Marja Hurley for research opportunities.
There are no paid openings available in the Hurley lab at this time, but volunteer positions are available.
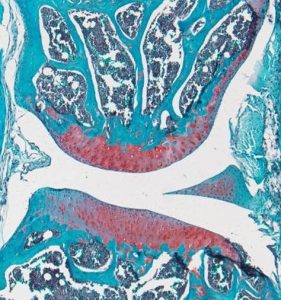
Dwarfism in HMW Transgenic Mice Phenocopy XLH
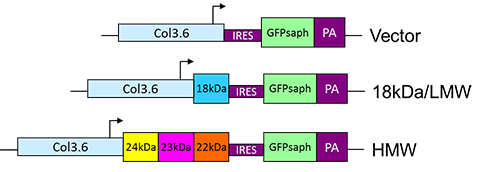
Schematic diagram of construction of 3.6Col-FGF2-IRES-GFPsaph plasmids.
Xiao and Hurley, J. Biol. Chem 2010.
FGF2 High Molecular Weight Isoforms Contribute to Osteoarthropathy in Male Mice
Knees of HMWFGF2Tg mice show changes in subchondral bone that mimic the progression of human osteoarthropathy in XLH subjects.
Meoburt and Hurley, Endocrinology 2016.
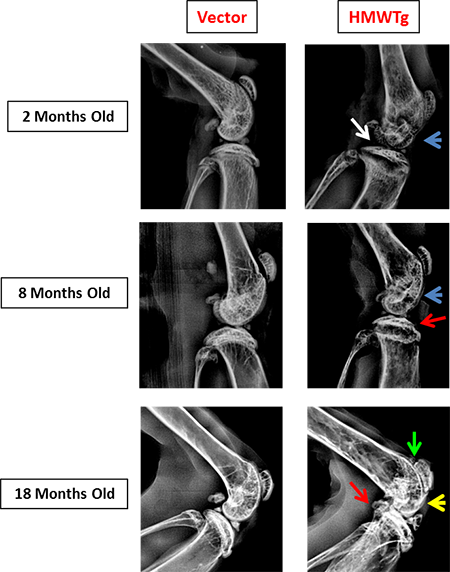
Red arrow: osteophyte formation
Blue arrow: thinning of subchondral bone
Yellow arrow: sclerotic bone
Green arrow: patellofemoral space narrowing
White arrow: flattening of tibial plateau
Sapphire GFP: TO-PRO-3 (Nuclear Stain)
No GFP expression in cartilage or tendon region.
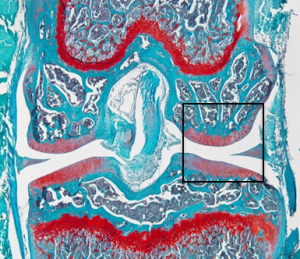
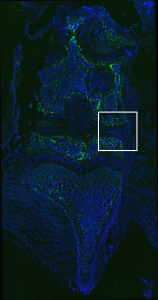
Vector
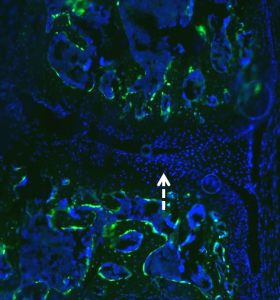
Vector
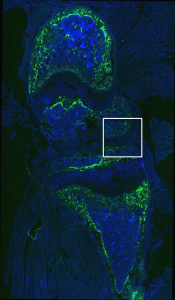
HMWTg
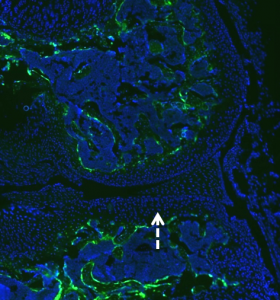
HMWTg
Vertebrae
Micro-CT of lumbar vertebrae from 2 months old HO mice showed BV/TV was markedly decreased in HMWTg mice compared with Vector mice.
Xiao and Hurley, J. Biol. Chem 2010.

Vector

HMWFG2Tg
Increased EDU Labeling of Proliferating Cells at Post Fracture Day 3 in Periosteum of Femurs of 12-Month-Old 18kDaFGF2Tg Mice
Mice were injected intraperitoneally with EdU 24 hours prior to sacrifice and fractured femurs were harvested and frozen sections were examined for EdU labeling. Proliferating nuclei were stained with DAPI. C=cortical bone, P=periosteum.

Vector

18kDa
Laser Capture Microdissection of Periosteal Area of Callus 7 Days Post Fracture
4% PFA persufion of mice, post fix for 2h. Embed in OCT. section at 7um on CryoJane.

Before LCM

After LCM

Captured Tissue