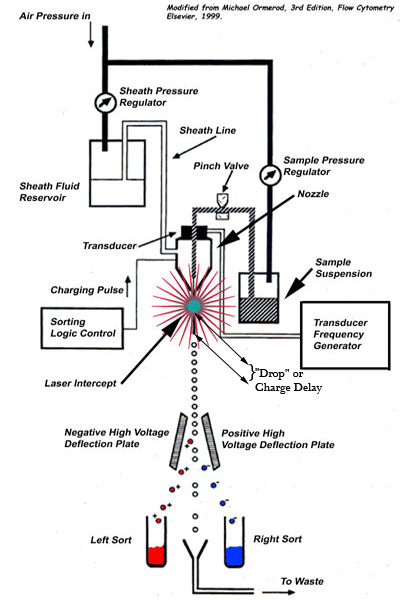
Flow cytometers utilize a sophisticated array of lasers, optics, fluidics and electronic detectors to measure light scatter and/or fluorescence emission from cells in purified suspension that are hydrodynamically focused to a single cell wide laminar flow column. The fluid column passes through laser light focused to a beam a few cell diameters across. Laminar flow permits laser interrogation of the cells in single file, one cell at a time. As each cell passes through the laser light it emits scattered light in all directions.
Light scatter measured at 90 degrees from incidence (right or orthogonal scatter) corresponds to the quantity and light diffractive quality of granular structures inside a cell, and light scatter measured at 180 degrees (forward scatter) approximately corresponds to cell size. These parameters alone are sufficient to distinguish numerous cell types and to exclude dead cells, aggregates and cell debris from cells of interest.
If the cells have been stained with fluorochromes (or transfected to express fluorogenic activity - GFP) they will also emit fluorescence intensities at levels which directly correspond to the density of fluorochrome on or within the cell. Notably, any cell structure for which an antibody or other ligand probe is available may be characterized with respect to surface or intracellular density by fluorescence emission. Any gene whose expression can be coupled to a fluor such as GFP can identify the cell in which it is expressed and be further characterized or sorted on that basis. Further thanks to steering optics and wavelength filters which retrieve light of various wavelengths and guide them to separate detectors, fluorescence intensities may be measured at several different wavelengths simultaneously for each cell, allowing multiple antigens to be measured.
In cytometers capable of sorting, the hydrodynamically focused cell stream is broken into uniform-sized droplets by axial vibration of the nozzle. A drop-charging signal is triggered by an electronic system that processes the fluorescent and scatter signals received from cells and determines the appropriate charge to give each cell. Only two populations can be retrieved since the uncharged cells go to waste. Some cytometers have overcome this limitation and can now separate up to four populations by producing four side streams by varying the charge applied to the drop. See U.S. Patent 5,483,469, January 9, 1996, to inventor Ger Van den Engh.