
Assistant Professor (Adjunct)
Department of Cell Biology
Contact
Email: shuhaibar@uchc.edu
UConn Health
263 Farmington Avenue
Farmington, CT 06030
As a Ph.D. student and postdoctoral fellow in the Jaffe lab, I investigated the regulation of oocyte meiosis in mammals, in particular the role of cyclic nucleotides in transmitting hormonal signals into the oocyte. In preovulatory ovarian follicles, meiotic arrest in the oocyte is maintained by cyclic GMP. Luteinizing hormone (LH) causes a decrease in cGMP, which initiates a cascade of signaling pathways inducing oocyte maturation in preparation for fertilization. How cGMP is lowered within the follicle is not completely understood.
Our research has contributed to our understanding of how luteinizing hormone acts in the follicle to restart oocyte meiosis in preparation for ovulation and fertilization. 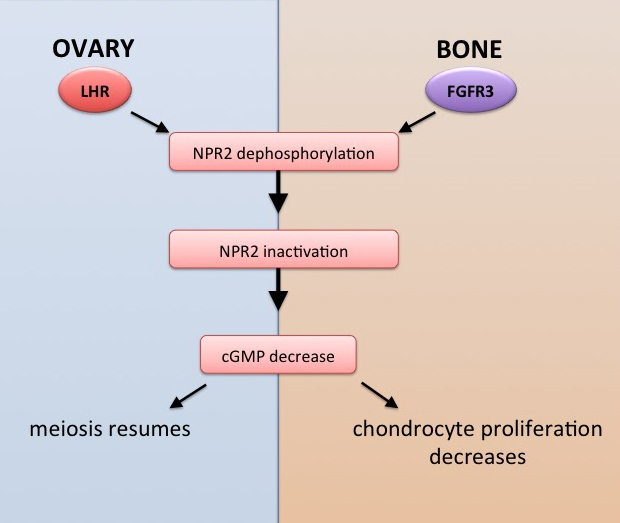 My research included developing new methods for live tissue microscopy, which I used to show for the first time that a physiological signal initiated by a stimulus in one region of an intact tissue can travel across many layers of cells via cyclic GMP diffusion through gap junctions (Shuhaibar et al., 2015). During the course of these studies, and in collaboration with Dr. Siu-Pok Yee (UConn Health) and Dr. Lincoln Potter (University of Minnesota), we developed a novel mouse model which mimics constitutively phosphorylated form of the guanylyl cyclase (NPR2) that is responsible for cGMP production in the ovary. These studies showed that the prevention of NPR2 dephosphorylation delays the normal progression of oocyte maturation (Shuhaibar et al., 2016).
My research included developing new methods for live tissue microscopy, which I used to show for the first time that a physiological signal initiated by a stimulus in one region of an intact tissue can travel across many layers of cells via cyclic GMP diffusion through gap junctions (Shuhaibar et al., 2015). During the course of these studies, and in collaboration with Dr. Siu-Pok Yee (UConn Health) and Dr. Lincoln Potter (University of Minnesota), we developed a novel mouse model which mimics constitutively phosphorylated form of the guanylyl cyclase (NPR2) that is responsible for cGMP production in the ovary. These studies showed that the prevention of NPR2 dephosphorylation delays the normal progression of oocyte maturation (Shuhaibar et al., 2016).
These discoveries established a new physiological mechanism that has major implications for understanding of other physiological processes as well, including the growth of bones. Bones in mice with constitutively active NPR2 are longer than bones in wildtype mice. Our experiments indicate that, just as LH signaling dephosphorylates and inactivates NPR2 in the ovary, fibroblast growth factor signaling dephosphorylates and inactivates NPR2 in the bone growth plate. These findings may have implications for development of new therapeutics for bone growth disorders such as achondroplasia (Shuhaibar et al., 2017; 2021). UConn Today article
Recent Publications
Shuhaibar, L.C., Kaci, N., Egbert, J.R., Horville, T., Loisay, L., Vigone, G., Uliasz, T.F., Dambroise,E., Swingle, M.R., Honkanen, R.E., Duplan, M.B., Jaffe, L.A., Legeai-Mallet, L. (2021) Phosphatase inhibition by LB-100 enhances BMN-111 stimulation of bone growth. JCI insight. 6 (9):141426.
Shuhaibar, L.C., Robinson, J.W., Vigone. G., Shuhaibar, N.P., Egbert, J.R., Baena, V., Uliasz, T.F., Kaback,D., Yee, S.P., Feil, R., Fisher, M.C., Dealy, C.N., Potter, L.R., Jaffe, L.A. (2017) Dephosphorylation of the NPR2 guanylyl cyclase contributes to inhibition of bone growth by fibroblast growth factor. eLife 6: e31343.
Shuhaibar, L.C., Egbert, J.R., Edmund, A.B., Uliasz, T.F., Dickey, D.M., Yee, S.P., Potter, L.R., and Jaffe, L.A. (2016) Dephosphorylation of juxtamembrane serines and threonines of the NPR2 guanylyl cyclase is required for rapid resumption of oocyte meiosis in response to luteinizing hormone. Dev Biol. 1;409(1):194-201. Supplement.
Shuhaibar, L.C., Egbert, J.R., Norris, R.P., Lampe, P.D., Nikolaev, V.O., Thunemann, M., Wen, L., Feil, R., and Jaffe, L.A. (2015) Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc. Natl. Acad. Sci. USA 112:5527-5532.