Ph.D. Biomedical Science Student
Phone: 860-679-2677
Email: inakashima@uchc.edu
I am a senior graduate student in the Ph.D. in Biomedical Science program. In the Jaffe lab, my research concerns signal transduction during oocyte maturation and fertilization.
LH-induced cyclic nucleotide changes involved in triggering meiotic resumption in the oocyte
My thesis project focuses on determining the kinetics of the luteinizing hormone (LH)-induced cyclic nucleotide changes and gap junction regulation required for the normal timing of meiotic resumption in mammalian preovulatory follicles. Previous studies have shown that in mammalian oocytes within preovulatory follicles, re-entry into meiosis is controlled by both cAMP and cGMP. However, it has been unclear how the kinetics of the LH-induced decreases in cGMP and cAMP compare. To address this, I am implementing a cGi500 FRET sensor and R-FLAMP1b sensor to measure oocyte cGMP and cAMP changes in response to LH. Using these sensors we’ve shown that in follicle enclosed-oocytes, LH causes a cGMP decrease, which precedes the cAMP decrease. (see poster from 2024 ASCB meeting). Additionally, I generated a new mouse line ubiquitously expressing cAMPFIRE-M, a Förster resonance energy transfer (FRET) sensor for cAMP to determine how oocyte cAMP levels can fall coincident with the LH-induced cAMP increase in the surrounding somatic cells. Using this mouse line, we’ve shown that LH elevates cAMP in the somatic cells, which diffuses through gap junctions and transiently increases oocyte cAMP.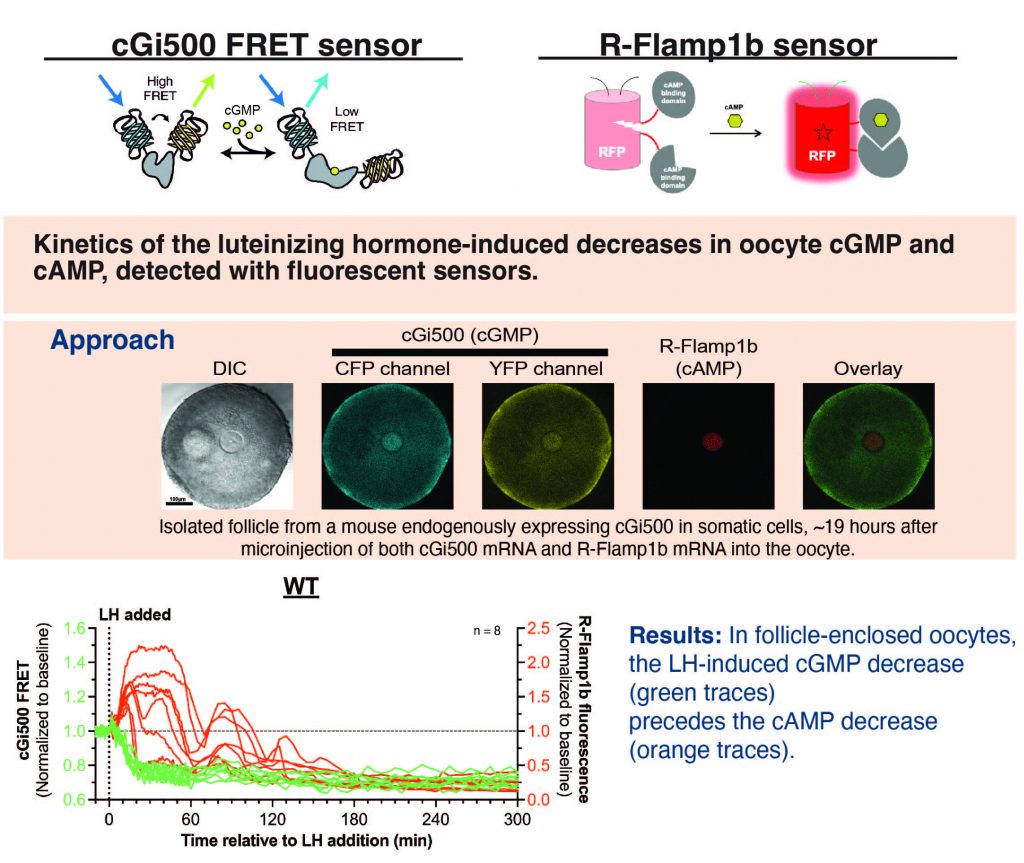
Visualization of sperm-egg interaction and fusion during fertilization in Arabidopsis
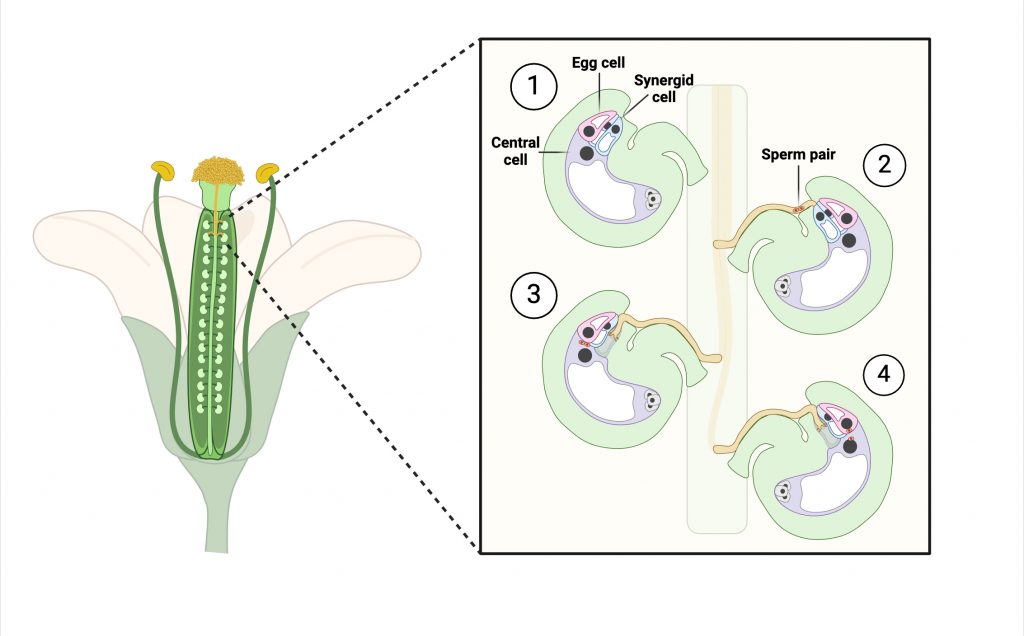
My secondary project focuses on sperm-egg fusion proteins involved in fertilization. At fertilization the sperm and egg plasma membranes adhere to each other, then fuse to form a zygote. HAPLESS2 (HAP2), a sperm-specific fusogen, has been discovered to be broadly conserved across several non-mammalian eukaryotic kingdoms (Fédry et al., 2018). HAP2 has a structure closely resembling viral fusion proteins (Fédry et al., 2018) and is required for sperm-egg fusion. Although studies have offered insight into HAP2-mediated fusion, ultrastructural information during sperm-egg fusion has been limited. To address this issue, we recently developed a method that implements live imaging and electron microscopy imaging, which has been useful for investigating sperm-egg interactions in Arabidopsis ovules (see poster from 2024 ICSPR meeting). Future directions include using this method to compare wild-type sperm with HAP2 knockout sperm and probing these samples with HAP2 antibodies to visualize HAP2 localization throughout the fusion process.
Fédry, J., Forcina, J., Legrand, P., Péhau-Arnaudet, G., Haouz, A., Johnson, M., Rey, F.A., Krey, T. (2018). Evolutionary diversification of the HAP2 membrane insertion motifs to drive gamete fusion across eukaryotes. PLOS Biology, 16(8): e2006357. https://doi.org/10.1371/journal.pbio.2006357
