Studying Rapid Processing of Electrical and Chemical Signals in Individual Neurons
Overview
Our laboratory's research is primarily focused on unraveling the cellular and molecular mechanisms underlying synaptic integration, synaptic plasticity, neuronal excitability, and the modulation of these fundamental processes by neuromodulators. We concentrate our efforts on neurons and synapses in the prefrontal cortex and neostriatum, key regions in the brain influencing voluntary movement, working memory, planning, and emotions. The prefrontal cortex is particularly intriguing due to its strong implication in major psychiatric diseases such as Alzheimer's disease and schizophrenia. Neostriatum is the largest segment of the basal ganglia – its functional deficits are manifested as Parkinson’s disease, Huntington’s disease, involuntary tics, and hallucinations (i.e. schizophrenia). Our experimental methodologies encompass local application or evoked release of neurotransmitters (glutamate, acetylcholine, and dopamine), electrophysiology (patch-clamp recordings), fast simultaneous multi-site imaging (using calcium-sensitive dyes, voltage-sensitive dyes, and genetically-encoded voltage indicators), computer simulations (NEURON), neuron tracing, and immunolabeling.
Voltage Imaging
Understanding the cellular mechanisms governing complex brain functions, including cognition and locomotion, necessitates monitoring membrane voltage at the cellular, circuit, and system levels. Seminal studies using voltage-sensitive and calcium-sensitive dyes have showcased the parallel detection of electrical activity across interconnected neuron populations in various preparations. The advent of genetically-encoded voltage indicators (GEVIs) holds tremendous promise for monitoring the formation and structure of neural ensembles. Compared to low-molecular-weight calcium and voltage indicators (dyes), GEVI imaging approaches are cell type-specific, less invasive, able to relate activity and anatomy, and facilitate long-term recordings of individual cell activities over weeks. This capability allows direct monitoring of the emergence of learned behaviors and underlying circuit mechanisms. We explore novel approaches based on GEVIs and compare them with other imaging techniques, including calcium imaging and voltage-sensitive dye imaging.
Alzheimer’s Disease
Alzheimer’s disease (AD) stands as the third most common ailment affecting the US population. AD is an age-dependent chronic neurodegenerative condition characterized by the accumulation of protein plaques in the brain, resulting in the loss of memory and cognitive capacity. Before the identification of brain plaques and observable behavioral changes, synaptic dysfunction (faulty synapses) begins to emerge, setting off a spiral of cognitive deterioration in AD patients. Intriguingly, inappropriate (imbalanced) electrical activity of neurons actually facilitates the accumulation of harmful protein plaques in the brain, the hallmark of AD pathology. Hence, AD is acknowledged as a disease of synaptic dysfunction. We propose the development of an optical imaging method to detect early changes in synaptic function occurring in animal models of Alzheimer’s disease.
Our Discoveries
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
We coined the term “Dendritic UP States” in 2005.
Analysis of the dendritic input–output function revealed that basal dendrites operate in a somewhat binary regimen (DOWN or UP) in regard to the amplitude of the glutamate-evoked electrical signal. The somatic plateau rises a few milliseconds after the onset of the dendritic transient and collapses with the breakdown of the dendritic plateau depolarization. In our in vitro model, the stable long-lasting somatic depolarization (UP state like) is a direct consequence of the local processing of a strong excitatory glutamatergic input arriving on the basal dendrite. The slow component of the somatic depolarization accurately mirrors the glutamate-evoked dendritic plateau potential (dendritic UP state).
A strict correlation between dendritic and somatic plateau depolarizations in the rat prefrontal cortex pyramidal neurons. Milojkovic BA, Radojicic MS, Antic SD. J Neurosci. 2005 Apr 13;25(15):3940-51. https://pubmed.ncbi.nlm.nih.gov/15829646/
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
We discovered the link between Dendritic Plateau Potentials and Neuronal UP States in 2004.
Burst generation in rat pyramidal neurones by regenerative potentials elicited in a restricted part of the basilar dendritic tree. Milojkovic BA, Radojicic MS, Goldman-Rakic PS, Antic SD. J Physiol. 2004 Jul 1;558(Pt 1):193-211. https://pubmed.ncbi.nlm.nih.gov/15155788/
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
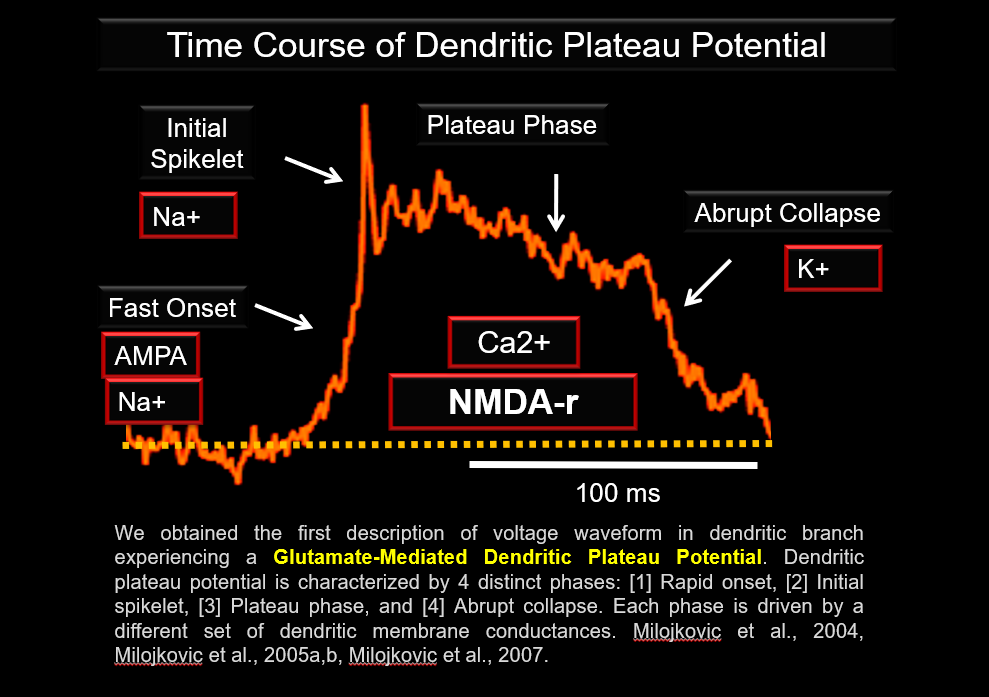
We obtained the first description of Voltage Waveform in dendritic branch experiencing a glutamate-mediated Dendritic Plateau Potential.
Dendritic plateau potential is characterized by 4 distinct phases, a rapid onset, initial spikelet, plateau phase, and abrupt collapse. Each phase is driven by a different set of dendritic membrane conductances. https://pubmed.ncbi.nlm.nih.gov/15155788/ & https://pubmed.ncbi.nlm.nih.gov/33085562/

Srdjan D. Antic, M.D.
Professor
Institute for Systems Genomics
Stem Cell Institute, Department of Neuroscience
UConn Health School of Medicine
263 Farmington Avenue, Room L-3052
Farmington, CT 06030
Phone: 860-679-8468
Email: antic@uchc.edu
Fax: 860-679-8766
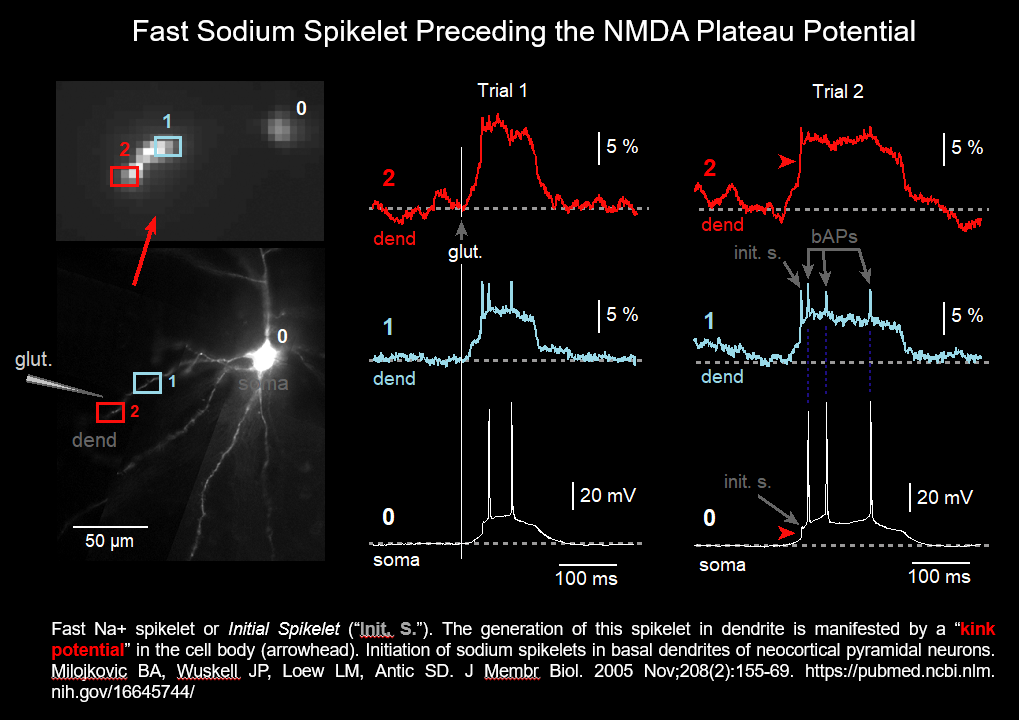
Dendritic Sodium Spikelet (2005)
The initial spikelet (shown in the figure above and in the figure below) is due to activation of voltage-gated sodium channels in thin dendritic branches (basal & oblique) of pyramidal neurons. Only 13% of basal dendrites generate local sodium spikelets.
Initiation of sodium spikelets in basal dendrites of neocortical pyramidal neurons. Milojkovic BA, Wuskell JP, Loew LM, Antic SD. J Membr Biol. 2005 Nov;208(2):155-69. https://pubmed.ncbi.nlm.nih.gov/16645744/
Local glutamate-mediated dendritic plateau potentials change the state of the cortical pyramidal neuron (2021) Peng P Gao, Joseph W Graham, Wen-Liang Zhou, Jinyoung Jang, Sergio Angulo, Salvador Dura-Bernal, Michael Hines, William W Lytton, Srdjan D Antic. J Neurophysiol. 2021 Jan 1;125(1):23-42. https://pubmed.ncbi.nlm.nih.gov/33085562/
AP Backpropagation in Thin Branches (2003 – 2009)
We obtained the first description of voltage waveforms occurring in thin dendritic branches (basal & oblique) during backpropagation of fast action potentials.
Action potentials in basal and oblique dendrites of rat neocortical pyramidal neurons. Antic SD. J Physiol. 2003 Jul 1;550(Pt 1):35-50. https://pubmed.ncbi.nlm.nih.gov/12730348/
Dynamics of action potential backpropagation in basal dendrites of prefrontal cortical pyramidal neurons. Zhou WL, Yan P, Wuskell JP, Loew LM, Antic SD. Eur J Neurosci. 2008 Feb;27(4):923-36. https://pubmed.ncbi.nlm.nih.gov/18279369/
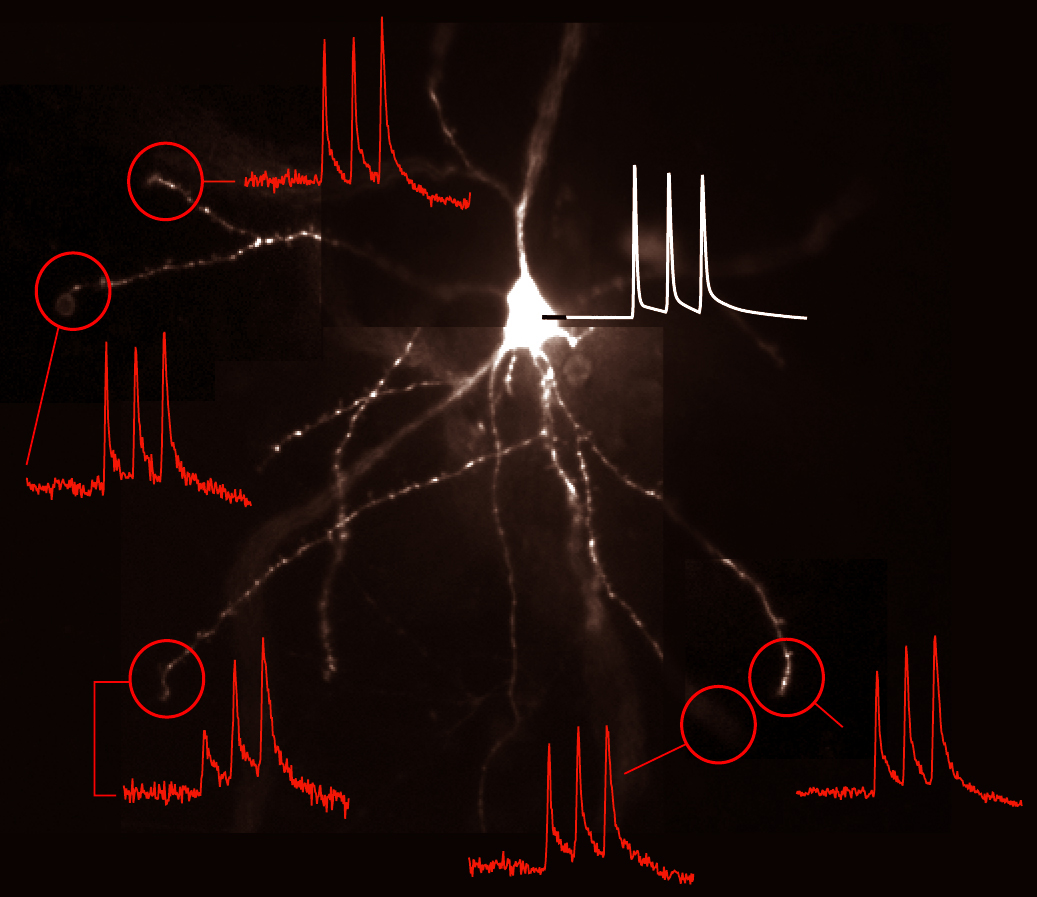
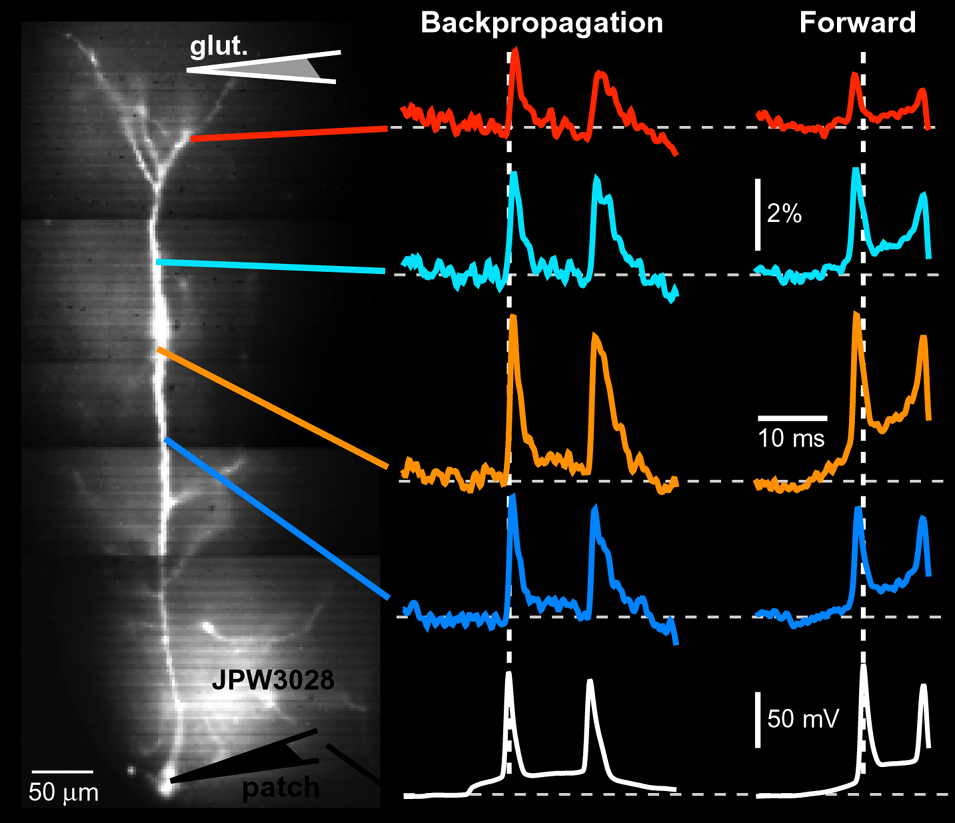
Voltage Recordings in Distal Tips of Several Basal Dendrites (2008 – 2021)

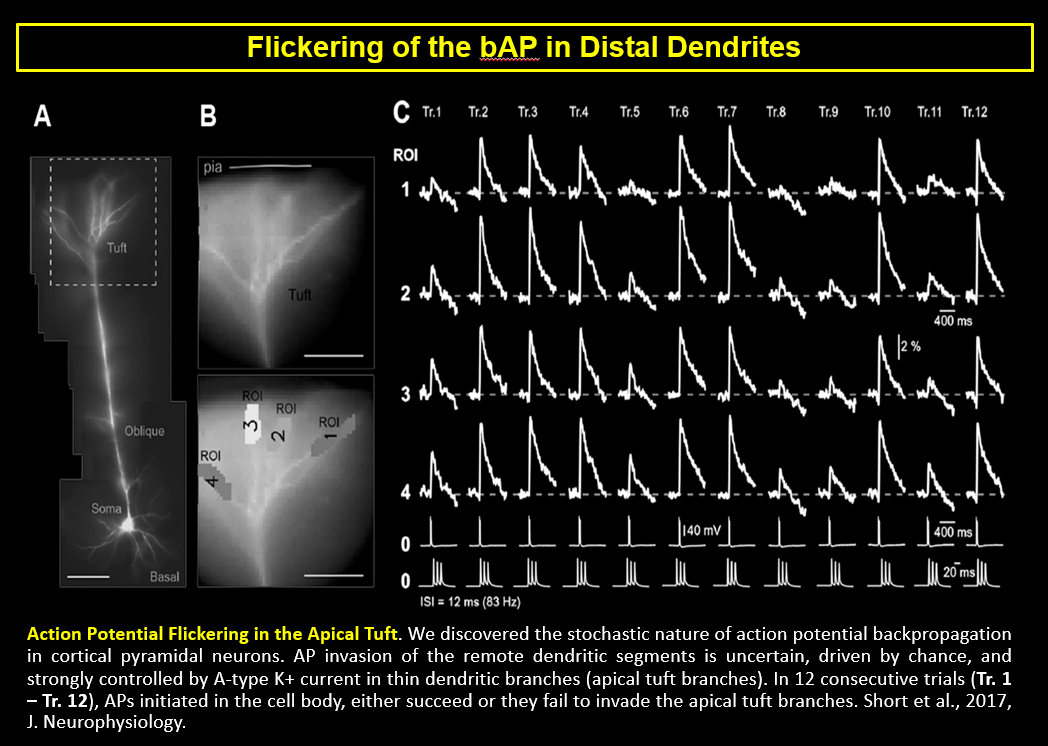
AP Flickering – Flickering of Backpropagating Action Potentials in the Apical Tuft (2017)
We discovered the stochastic nature of action potential backpropagation in cortical pyramidal neurons. AP invasion of the remote dendritic segments is uncertain, driven by chance, and strongly controlled by A-type K+ current in thin dendritic branches (apical tuft branches). https://pubmed.ncbi.nlm.nih.gov/28566465/
Spike-Order-Dependent Action Potential Backpropagation (2017)
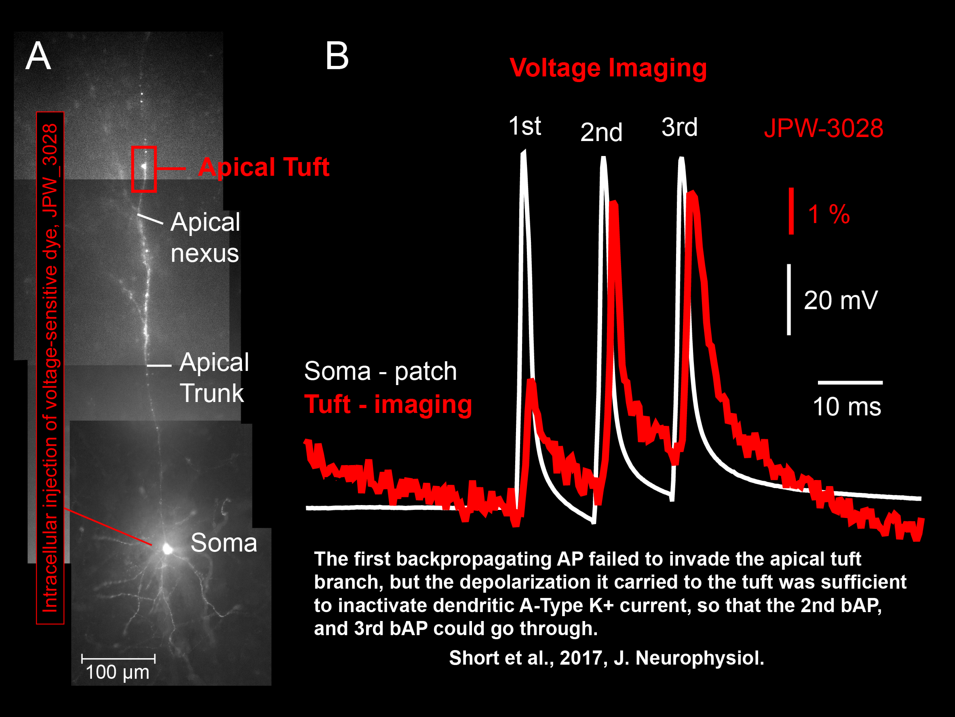 Shaina M Short, Katerina D Oikonomou, Wen-Liang Zhou, Corey D Acker, Marko A Popovic, Dejan Zecevic, Srdjan D Antic (2017) The stochastic nature of action potential backpropagation in apical tuft dendrites. J Neurophysiol. 2017 Aug 1;118(2):1394-1414. https://pubmed.ncbi.nlm.nih.gov/28566465/
Shaina M Short, Katerina D Oikonomou, Wen-Liang Zhou, Corey D Acker, Marko A Popovic, Dejan Zecevic, Srdjan D Antic (2017) The stochastic nature of action potential backpropagation in apical tuft dendrites. J Neurophysiol. 2017 Aug 1;118(2):1394-1414. https://pubmed.ncbi.nlm.nih.gov/28566465/
For more information about experiments in which transient depolarizations of dendritic segments inactivates local A-type K current and improves action potential backpropagation efficacy, see papers published by Daniel Johnston’s Lab (Texas, USA). K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Hoffman DA, Magee JC, Colbert CM, Johnston D. Nature. 1997 Jun 26;387(6636):869-75. doi: 10.1038/43119.