Research
The Yan Lab Studies the Pathogenesis and Treatments of Alzheimer’s Disease.
Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases, and our lab aims to understand AD pathogenesis in the brain and to identify therapeutic treatments by focusing on three research areas.
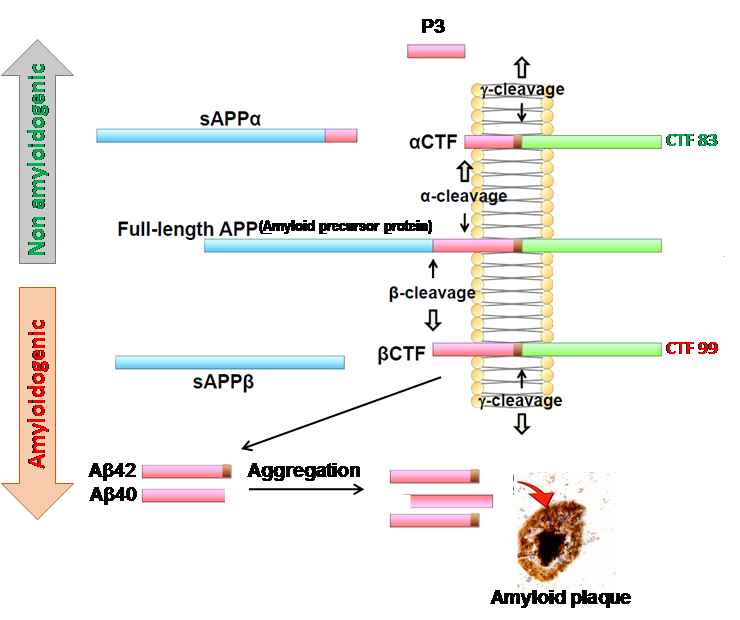
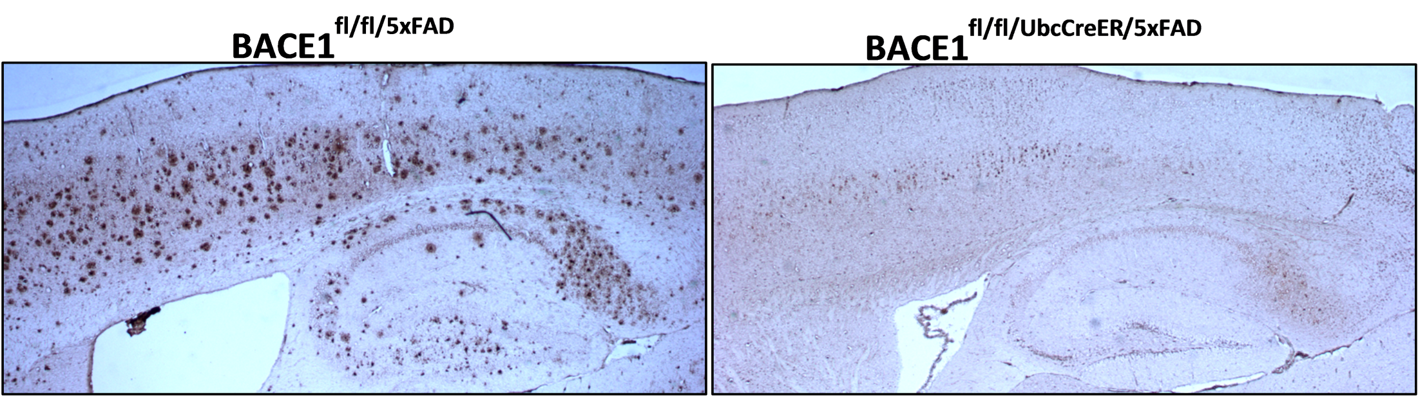
One of our research areas investigates the formation of extracellular amyloid-β (Aβ) plaques in the brain, which is an early event in the pathogenesis of AD that leads to a cascade of neurological dysfunction and dementia. The beta secretase 1 (BACE1) enzyme (discovered in the Yan Lab) initiates the generation of this toxic Aβ peptide through β-cleavage of amyloid precursor protein (APP) (Figure 1). We believe that precise inhibition of BACE1 could reduce toxic levels of Aβ safely. Toward this goal, we employ various in vitro and in vivo assays to determine the physiological function of BACE1 so that drugs targeted for BACE1 inhibition will be safe and effective for AD patients. Our recent study showed that progressively increased deletion of BACE1 in an adult AD mouse model reverses amyloid deposition (Figure 2). This reversal in amyloid deposition also resulted in significant improvements of Aβ-mediated synaptic dysfunctions.
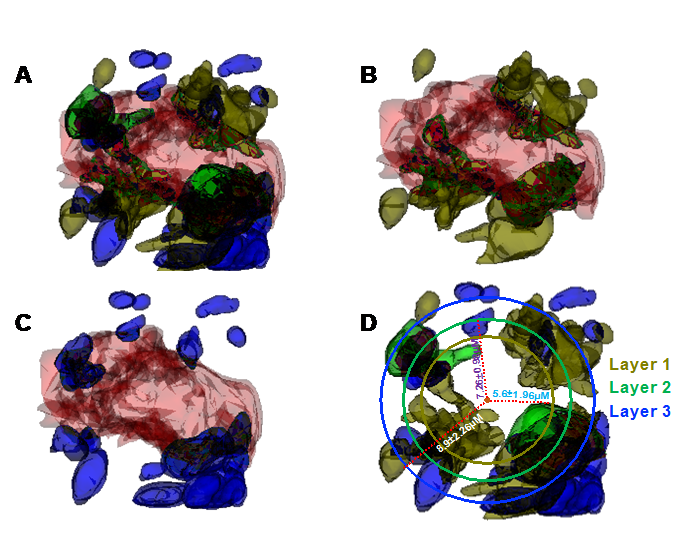
Our second research area investigates the mechanism of dystrophic neurite (DN) formation in the brain. DNs are swollen neuritic processes and their presence reflects synaptic impairments. Although amyloid deposition is also found in non-demented aging brains, often in diffuse forms, senile plaques in AD brains mainly consist of the amyloid core surrounded by reactive glial cells and DNs. We discovered that DNs in AD brains are formed sequentially, involving disruption of various cellular vesicles such as the tubular endoplasmic reticulum (marked by the reticulon-3 [RTN3] protein), pre-autophagosomes, and mature phagosomes (Figure 3). Further studies are aimed at delineating the molecular linkages between these cellular vesicles in AD brains.
Our third project is exploring approaches for reversing neuronal loss in AD brains, a common feature of late stage AD pathogenesis. We identified a protein, C-X3-C Motif Chemokine Ligand 1 (CX3CL1), that enhances neurogenesis in adult mouse brains, and we hypothesize that promoting regeneration of neurons may replenish degenerated neurons in AD patients’ brains. We are currently testing this hypothesis in AD mouse models.
Figure 1: Amyloid precursor protein (APP) is a large type 1 transmembrane protein that is normally cleaved by α-secretase (α-cleavage) and produce a soluble α-fragment (sAPPα) and a membrane bound c-terminal fragment (αCTF or CTF-83) (non-amyloidogenic pathway). A further cleavage on αCTF by γ-secretase generate a 3 kDa protein (P3) that normally degrades. In amyloidogenic pathway of APP metabolism, first cleavage of APP is initiated by β-secretase, known as beta amyloid cleaving enzyme 1 or BACE1, which generate a soluble sAPPβ fragment and a membrane bound CTF-99 (also called βCTF) fragment. A second cleavage on CTF-99 by γ-secretase on membrane generate β-amyloid (Aβ) peptides. The differential cleavage of γ-secretase may produce variable length of Aβ from 38 to 43 amino acid (aa) long, among those Aβ42(1-42aa long) and Aβ40 (1-40aa long) are the most prominent forms. Aβ peptides undergoes spontaneous aggregation, and Aβ42 is more prone to aggregates compare to Aβ40. A sequential aggregation and conformational changes from monomer > small oligomers>large oligomers >protofibrils>fibrils and their extracellular deposition develop Aβ plaque. In Alzheimer’s disease patients’ brain, such an Aβ plaque is often surrounded by dystrophic neurites, activated microglia and astrocytes.
Figure 2: Fixed brain sections from AD mouse (BACE1fl/fl/5xFAD) and BACE1 deleted AD mouse (BACE1fl/fl/UbcCreER/5xFAD) were stained with antibody 6E10 to label amyloid plaques. A high load of amyloid plaques was observed in ten-month old BACE1fl/fl/5xFAD mice, while no amyloid plaques were observed in age-matched BACE1fl/fl/UbcCreER/5xFAD mice.
Figure 3: Reconstructed three-dimensional (3D) structures of three types of dystrophic neurites (DNs) and their layer organization in 5xFAD mice brain. A 3D structure of a whole plaque was reconstructed through a stack of 225 3D electron microscopic images from a 10-month-old 5xFAD mouse brain using Reconstruct software. The distances of small vesicles (type I DNs; dark olive-green color), large double-membrane vesicles (type II DNs; blue color), and endoplasmic reticulum (ER) tubule–mitochondria inclusions (type III DN/RTN3 immunoreactive DNs or RIDNs; green color) from the center of the amyloid core (deep orange color) were measured on each of 225 images. (A) The 3D structures show the distribution of the three types of DNs, including many small vesicles (type I DNs) embedded in the amyloid core (B) and larger vesicles (type II DNs) being located further from the core (C) compare with type I DNs. A circle was drawn for each type of DN and their calculated average distances from the center of the amyloid core is shown (D). The 3D reconstructed structure shows amyloid core encircling DNs are constituted in three sequential layers, in which the first layer is established with type I DNs (small vesicles), followed by a second layer with type III (clustered tubular ER), and finally a third layer with type II (defective autophagy vesicles) DNs.