Current Research Projects
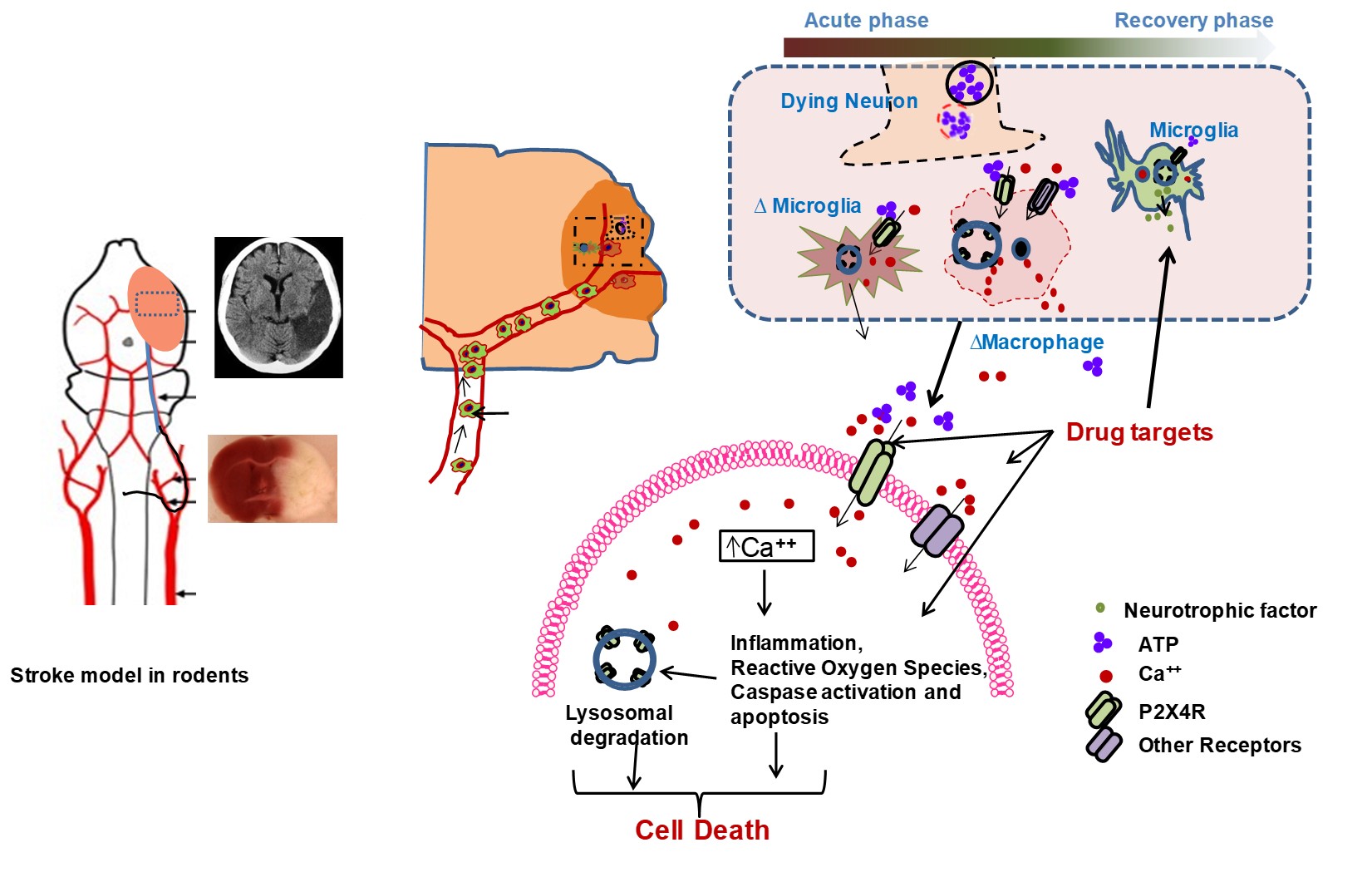
Role of Purinergic receptor P2X4 in stroke injury
Following a stroke, a surge of ATP is released from compromised brain cells. This surge, in turn, triggers a cascade of events, including the activation of neurons and microglial purinergic receptor P2X4 (P2X4R). This activation facilitates rapid excitatory neurotransmission through the influx of cations. However, excessive activation of P2X4R can lead to the release of several pro-inflammatory cytokines during the initial stages of ischemic injury. Interestingly, the effects of acute activation stand in contrast to those observed with chronic inhibition or the absence of this receptor. In fact, prolonged inhibition or the lack of P2X4R might hinder the process of stroke recovery. Thus, acknowledging the dual role of P2X4R in different phases of ischemic injury, we are currently engaged in a systematic exploration of its potential as a therapeutic target for enhancing post-stroke recovery. In essence, our research endeavors revolve around deciphering the intricate role of P2X4R, recognizing its potential as a double-edged sword in stroke-induced processes. Through a comprehensive understanding of its temporal dynamics, we aim to pave the way for innovative therapeutic interventions that can tip the balance in favor of improved recovery outcomes.
Stroke model and P2X4R mediated immune infiltration in ischemic stroke
Encephalomyosynangiosis for stroke recovery
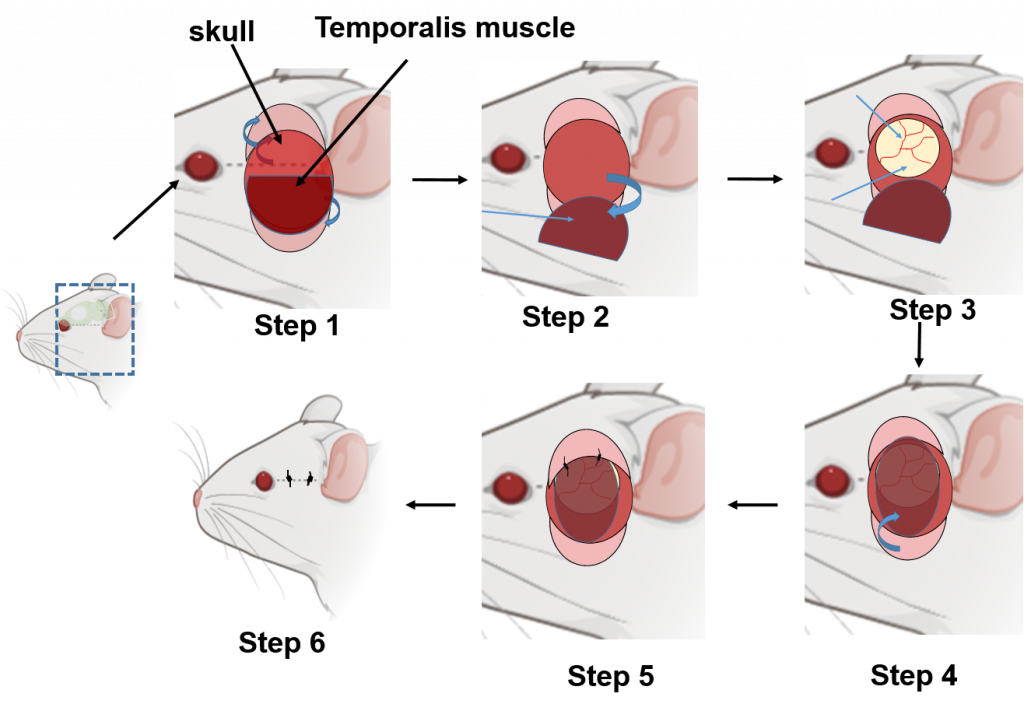
Encephalomyosynangiosis (EMS) is a neurosurgical procedure with low morbidity that is applied to promote collateral vascular formation in patients with moyamoya disease, a condition with progressive narrowing of cranial arteries and consequent low blood flow that increases risk for ischemic stroke. The procedure involves placement of a temporalis muscle flap on ischemic brain tissue. Human data suggest that Significant collateral formation emerges within months following EMS in individuals with moyamoya disease. This observation sparked a hypothesis suggesting that EMS, owing to its local and robust tissue nature, serves as a wellspring of vascular endothelium and angiogenic growth factors. These factors are believed to foster angiogenesis, thereby potentially bolstering neuronal survival after ischemic stroke.
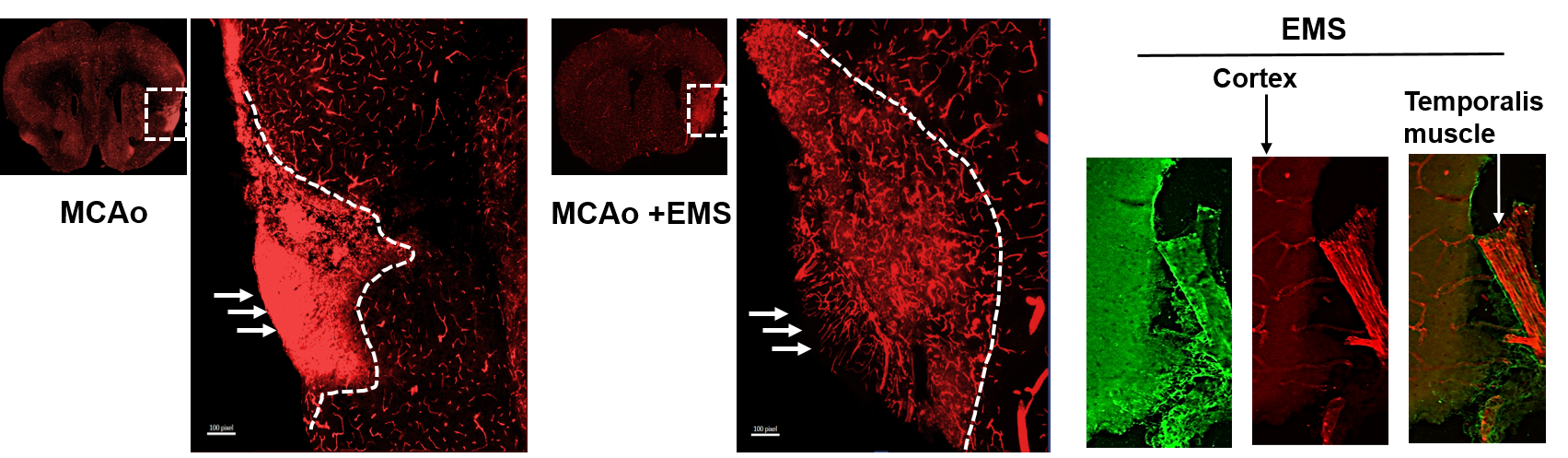
To validate this notion, we embarked on a groundbreaking venture, introducing EMS surgery to mice for the first time post ischemic stroke. Preliminary findings from this experimental model reveal a promising connection: the EMS surgery’s temporalis muscle graft successfully integrated with the cortical surface within 21 days. Impressively, mice receiving EMS subsequent to stroke exhibited a noteworthy surge in lectin-positive blood vessel formation compared to their stroke counterparts lacking EMS intervention. These initial results highlight the potential of EMS as a safe and feasible strategy to rejuvenate blood supply to ischemic tissue. However, we acknowledge the need for more comprehensive, extended investigations to solidify these promising outcomes.
This innovative model serves as a pivotal platform to delve deeper into the potential of EMS for ischemic stroke treatment. The overarching objectives encompass assessing EMS’s role in promoting post-stroke angiogenesis outside the realm of moyamoya disease and discerning if EMS can drive sustainable long-term functional recovery following non-moyamoya ischemic stroke.
Testing novel miRNA inhibitors for the treatment of stroke
MicroRNAs (miRNAs) represent a class of short, non-coding RNAs that have emerged as a potent tool for therapeutic intervention across various diseases, including stroke. Operating as intricate molecular orchestrators, miRNAs wield their influence by intricately adjusting protein expression levels and regulating gene expression. Their unique ability to simultaneously target multiple players within pathways associated with stroke pathology underscores their significance.
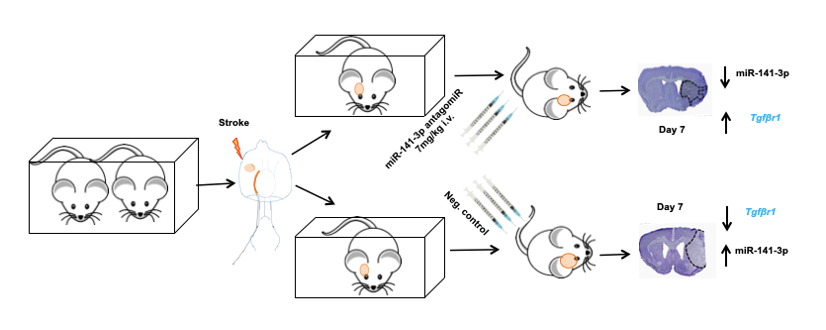
Within this project’s framework, our attention is fixed on the dynamic landscape of miRNA expression in post-stroke mice. The central objective revolves around investigating the potential impact of manipulating these miRNAs—either by blocking them through genetic deletion or antagomirs, or by enhancing their presence via mimics. This exploration aims to illuminate the role of target miRNAs and how their modulation can shape the aftermath of stroke. Moreover, our endeavors extend towards the realm of innovation. We are actively engaged in the development of a novel therapeutic approach—leveraging gamma PNA (peptide nucleic acid)—to inhibit miRNAs. This pioneering avenue holds the promise of refining post-stroke functional recovery, marking a potentially transformative step forward in stroke treatment strategies. In essence, our project unravels the intricate symphony orchestrated by miRNAs, exploring their potential as agents of change in the landscape of stroke intervention. Through careful investigation and creative innovation, we aspire to pave the way for new dimensions of post-stroke recovery, offering hope and healing to those affected by this condition.
Schematics in vivo work flow after treatment with miRNA inhibitor in mice after ischemic stroke