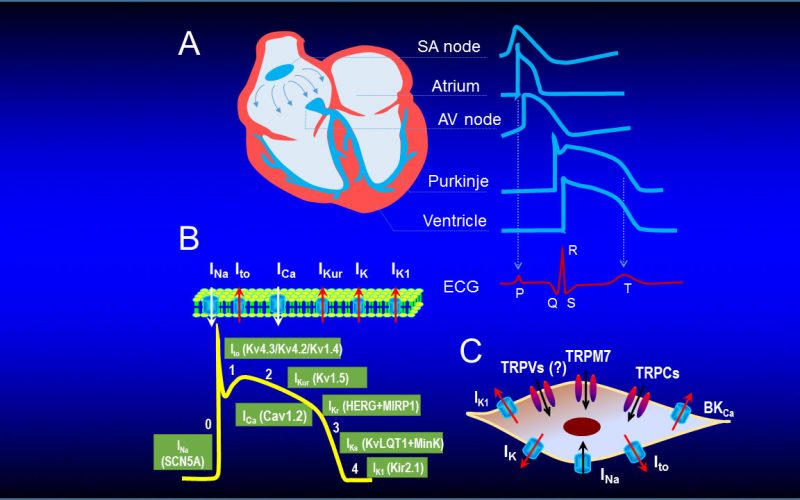
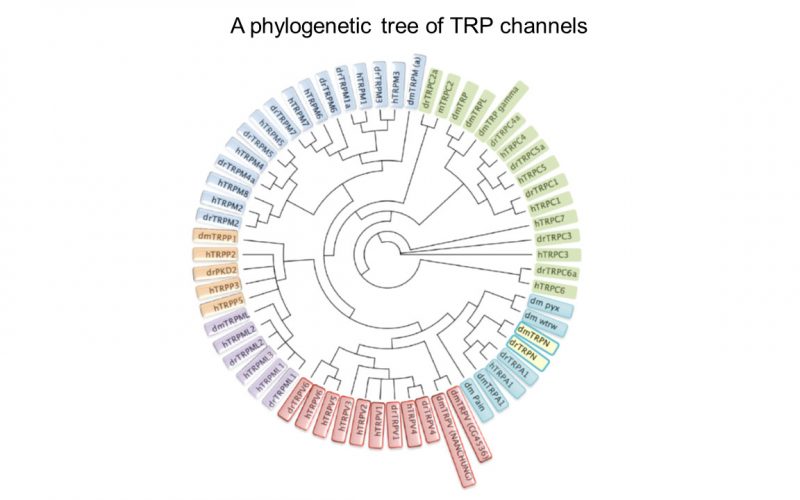
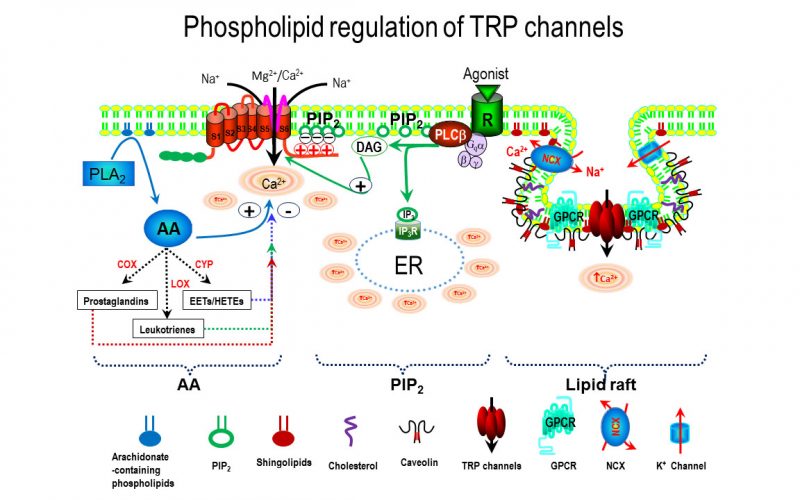
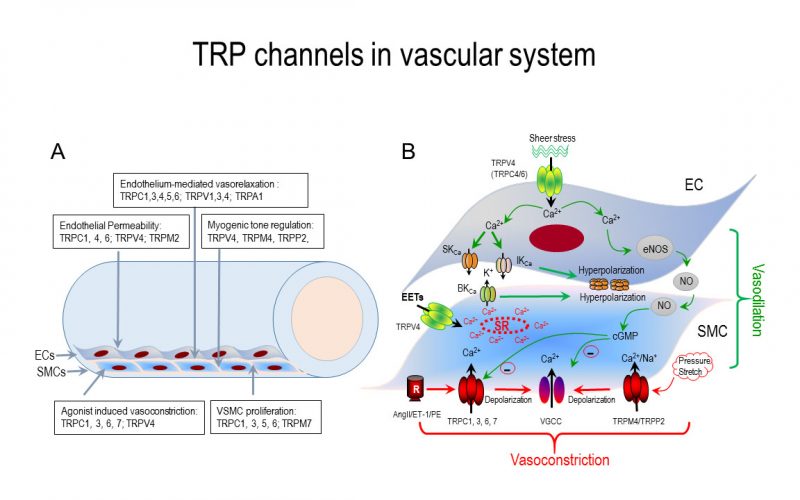
Calcium is the most common signal transduction element in virtually all cells ranging from bacteria to neurons. Recent studies have demonstrated the importance of transient receptor potential (TRP) channels in mediating calcium signals. The mammalian TRP channel superfamily consists of a diverse group of calcium permeable non-selective cation channels that may play a role in pain transduction, thermo-sensation, mechanotransduction, tumor suppression, vasodilatation, and neurodegenerative disorder. Twenty-eight mammalian TRP channel genes have been cloned since the first TRP channel protein was identified in Drosophila, yet their physiological functions are to be revealed.
The goal of our research is to understand the fundamental pathogenic mechanisms of cardiovascular diseases and ischemic brain injury, and to identify better therapeutic targets. Using multidisciplinary state-of-the-art techniques such as mouse genetics, electrophysiology, ratio calcium imaging, confocal microscopy, molecular biology, CRISPR, and bioinformatics, our research is to reveal the role of TRP channels in the initiation and progression of fibrotic heart disease as well as ischemic injury in the heart and brain.
Biophysics of Ion channels
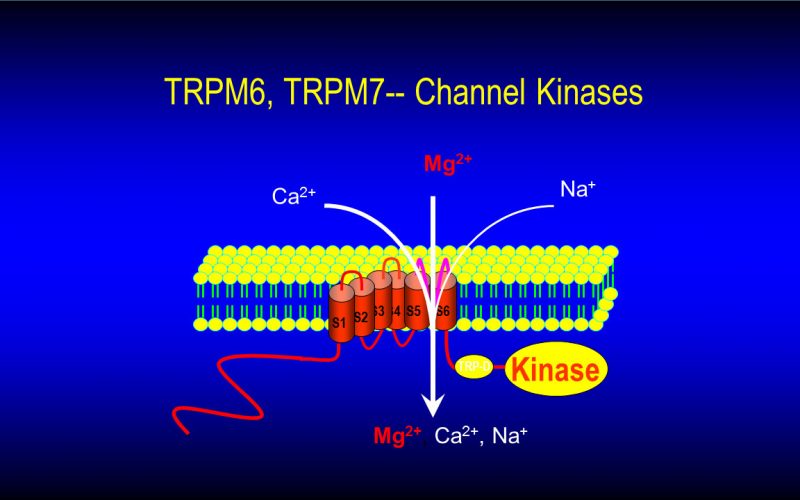
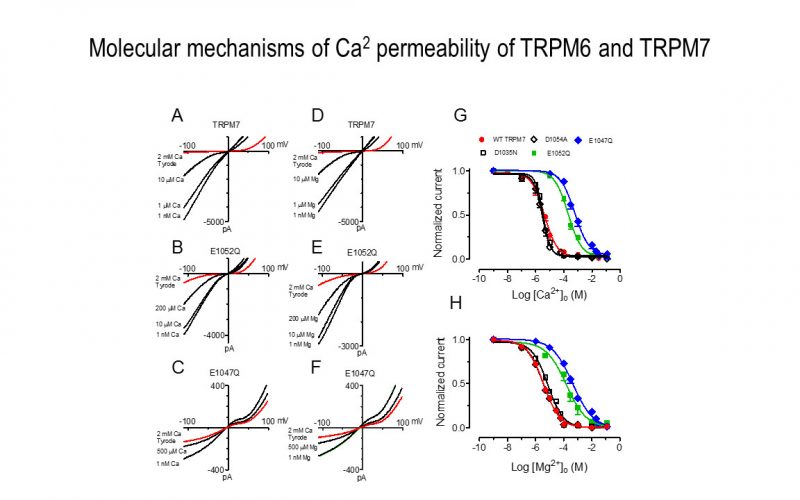
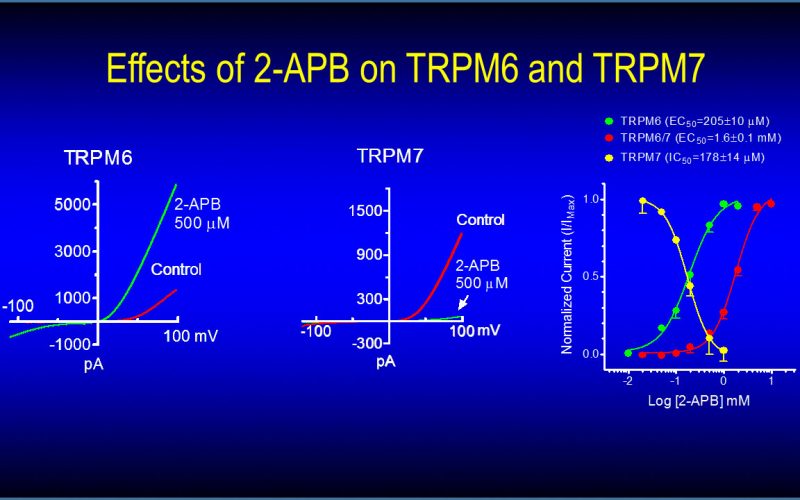
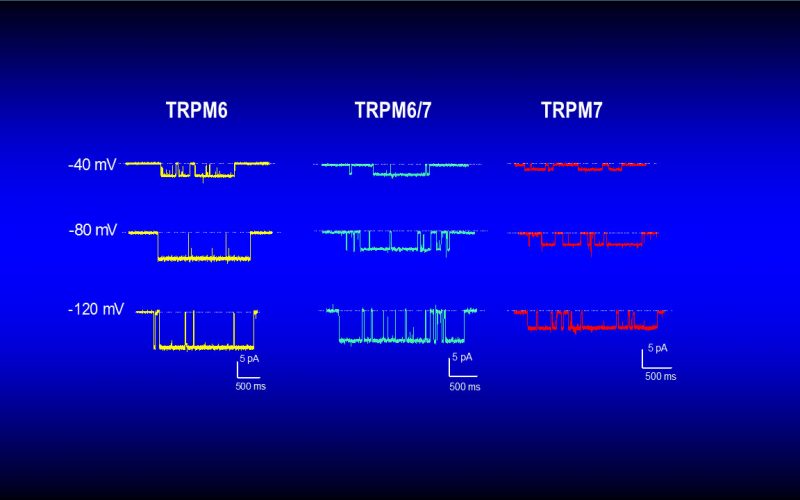
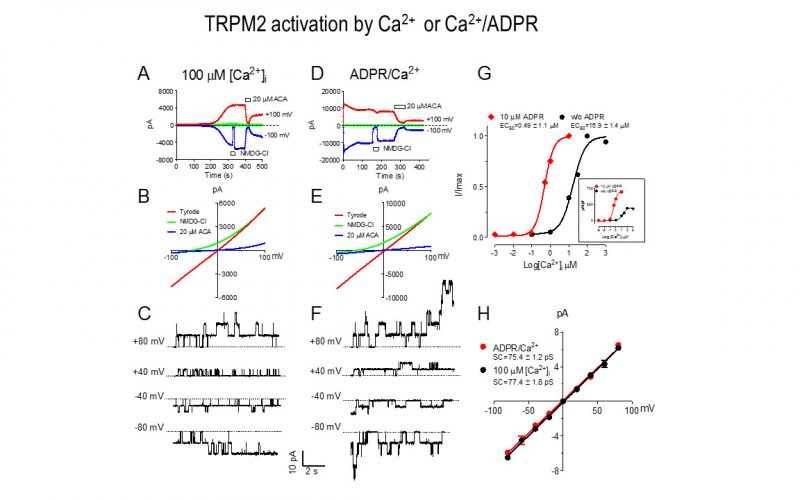
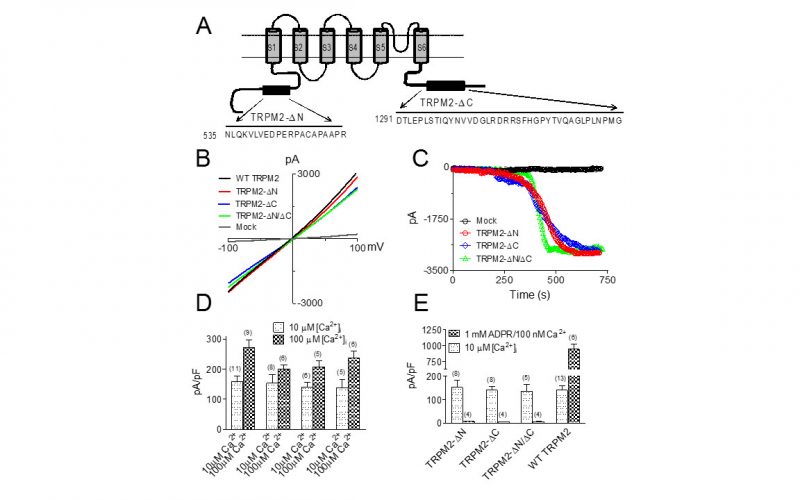
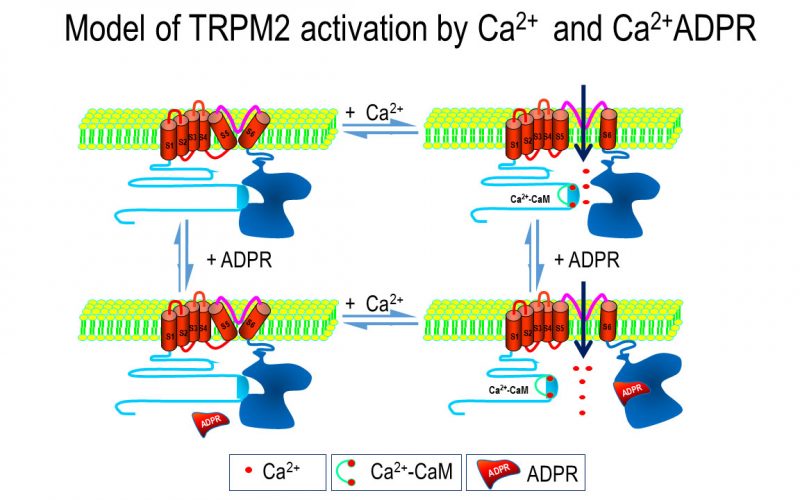
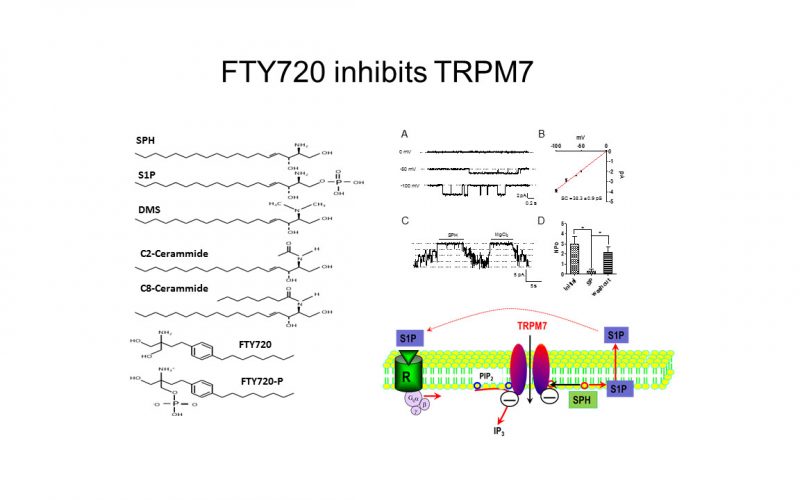
To understand the physiological/pathological functions of ion channels, we first need to understand how and when the channels open and close. Using mutagenesis and patch-clamp in heterologous expression system, we having been focusing on how the channel-kinases TRPM6 and TRPM7 are activated, and how the oxidative stress-sensitive TRPM2 is regulated by physiological and pathological conditions.
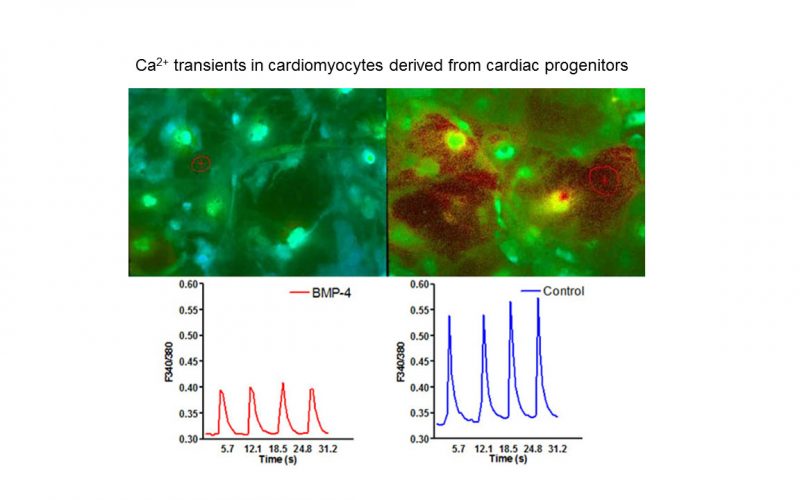
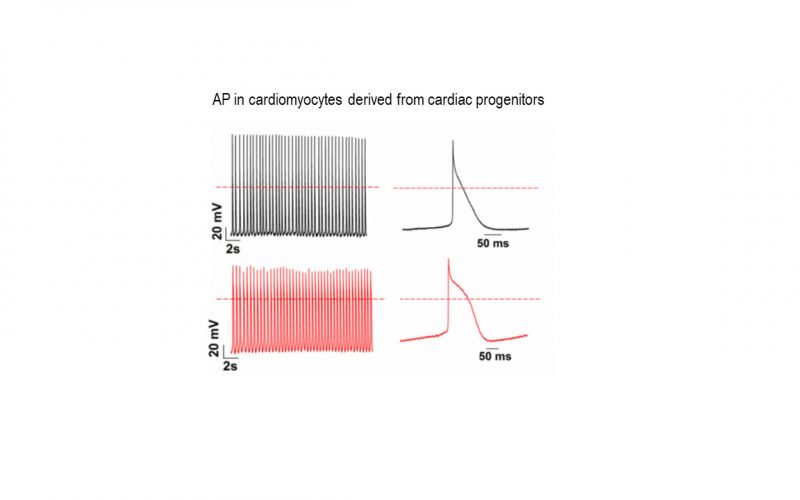
TRP channels and calcium signaling in fibrotic heart diseases
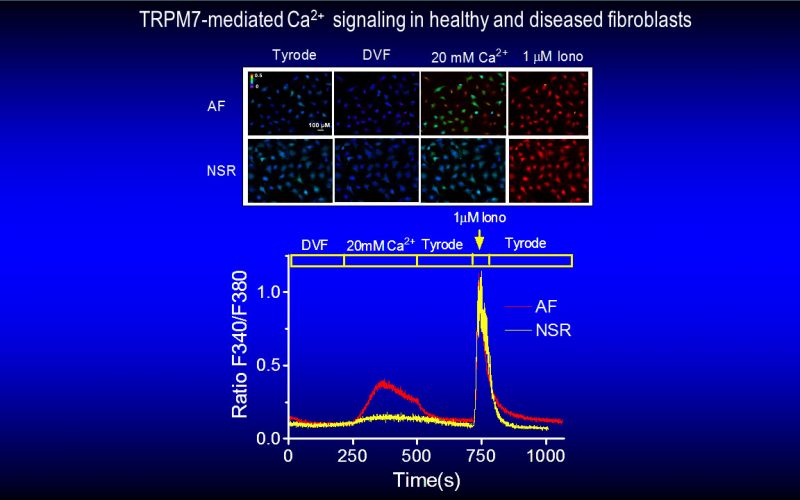
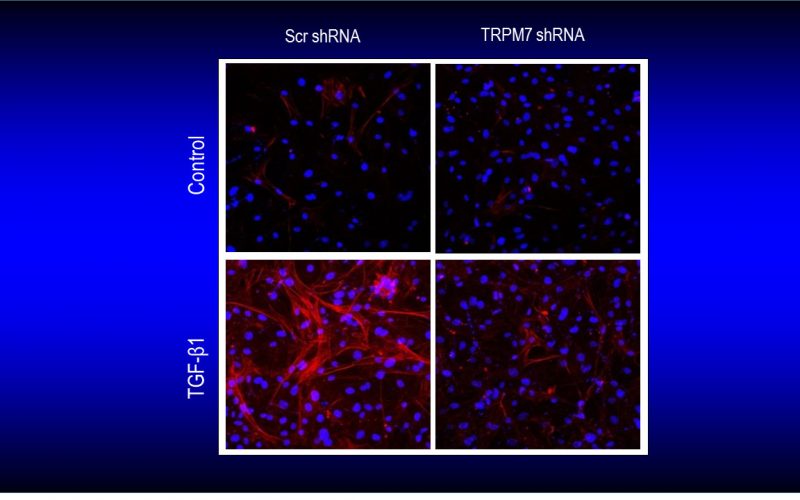
Fibrosis is a detrimental factor for various diseases in different organs. In the hearts, cardiac fibrosis is associated with hypertrophy, heart failure and arrhythmia. We have recently found that TRP channel-mediated Ca2+ signal is essential for cardiac fibrogenesis. To understand whether TRP channels especially TRPM7 is a potential therapeutic target for fibrotic heart diseases, we have established various platforms including conditional knockout and knockin mice, hypertensive heart failure mouse model, and ischemic heart failure mouse model. We use state-of-the-art techniques such as in vivo pressure-volume analysis, echocardiograph, and working heart to assess heart functions in order to understand how TRP channels play a role in the pathogenesis of fibrotic heart diseases.
Role of TRP channels in ischemic injury in the heart and brain
Ischemic injury causes not only acute damage including mortality but also long-term remodeling leading to chronic diseases. Although the consequence of ischemia and progression of chronic remodeling after ischemia may differ in different tissues and organs, the common cause of ischemic injury is the inflammation of vasculature. One focus of our research is to understand the mechanisms of systemic inflammation by investigating the role of TRP channel-mediated Ca2+ signaling in the pathogenic cascade. We use ischemic brain stroke mouse model to evaluate the role of TRP channels in systematic inflammation and the subsequent ischemic injury in the brain. We believe that identification of an ideal target for systemic inflammation and ultimately development of a therapeutic approach for systemic inflammation will largely reduce ischemia in various organs and therefore can be preventive for ischemic injury caused mortality and morbidity.