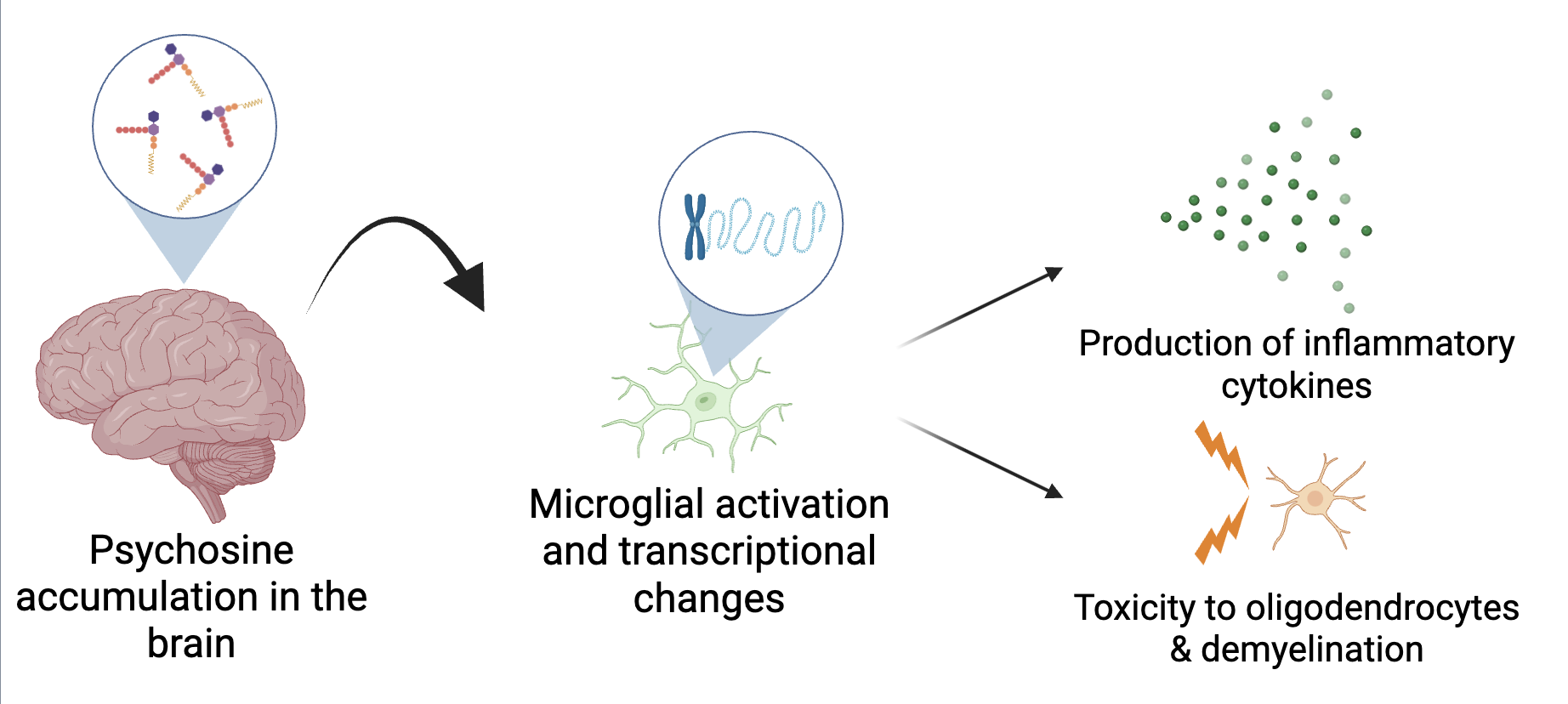
Microglial pathology is a crucial contributor to the progression of Globoid Cell Leukodystrohpy (GLD, also known as Krabbe Disease). Globoid cells, which are highly activated and multinucleated phagocytes, form prenatally before any demyelination or oligodendrocyte cell death occurs (Martin et al., 1981). Our lab has previously suggested that globoid cell formation is a microglial response to psychosine accumulation in the brain and could be a primary initiating step of GLD disease (Ijichi et al., 2013). Extensive microglial activation, as measured by elevated expression of activation markers like iba1 and cd86, is observed as early as postnatal day (P) 14 in the twitcher (twi) mouse (Sutter et al., 2023). Similarly, production of pro-inflammatory cytokines by microglia mediate microglial cytotoxicity to oligodendrocytes, in addition to the recruitment of peripheral immune cells (Merrill et al., 1993). More recently, microglia have been highlighted for their importance in GLD disease progression. A 2025 study indicated that replacement of twi microglia in the twi brain with wild-type (WT) microglia doubled survival of the twi mouse and significantly reduced demyelination (Aisenberg et al., 2025). Thus, identifying drivers of these pro-inflammatory microglial functions is significant for better understanding the pathology of this disease (Figure 1).
Figure 1: Hypothesized mechanism by which psychosine drives microglial activation and downstream neuroinflammation and demyelination.
Our lab has previously interrogated the transcriptomic differences in microglia from WT and twi brains at P21 via single-cell RNA sequencing. This dataset identified a significant number of differentially-expressed genes in the twi brain that are known to be regulated by the epigenetic regulator Disrupter of telomeric silencing 1-like (DOT1L) . DOT1L is a ubiquitously expressed histone methyltransferase that is capable of both activation and repression of transcription via methylation of histone 3 lysine 79 (H3K79). Immunohistochemistry for the DOT1L methylation mark H3K79me2 and Cd11b verified that DOT1L activity was significantly elevated in Cd11b+ cells in P30 Twi mouse brains as compared to age-matched WT samples (Figure 2). These data suggest that DOT1L may be a key regulator of microglial function in this disease that is worth investigating further. Thus, we hypothesize that microglial DOT1L activity contributes to neuropathology, including demyelination, in the twi mouse.
Figure 2: Immunohistochemical analysis of P30 twi brains reveals elevated DOT1L activity in microglia.
Current studies are characterizing the effects of DOT1L activity on microglial transcriptional networks. Further, the role that DOT1L activity in microglia plays in demyelination and oligodendrocyte cytotoxicity is being investigated through both pharmacologic inhibition of DOT1L and a transgenic mouse model. The findings of this study will expand upon our understanding of GLD disease pathology, where dysregulation of transcriptional networks is a key feature contributing to disease.