2026
January 2026
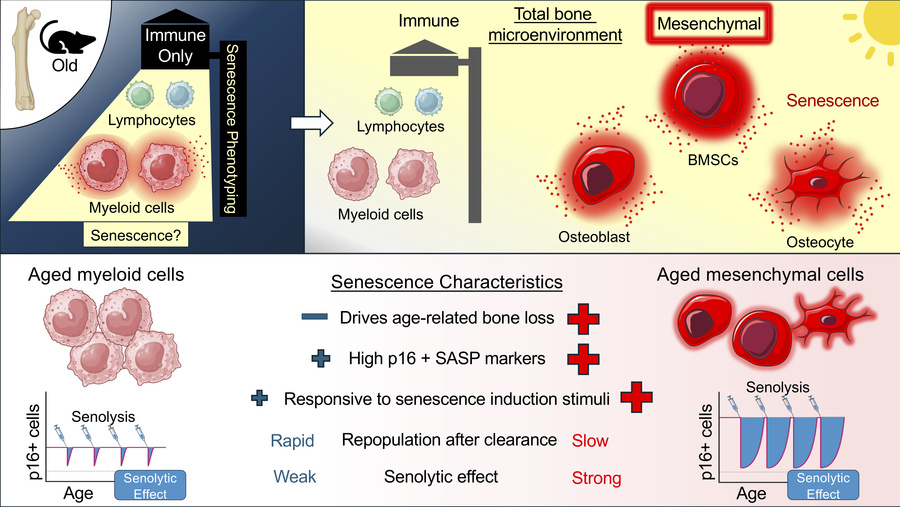
Mesenchymal versus myeloid senescence paper is online
This work, published in the Journal of Clinical Investigation, studies the different senescence phenotypes of myeloid cells versus mesenchymal cells in the aged bone microenvironment and their contributions (or lack thereof) to age-related bone loss. These results suggest that bone marrow myeloid cells do not develop a chronic senescence phenotype akin to mesenchymal bone and marrow cells.
Aged murine bone marrow myeloid and mesenchymal cells develop unique senescence phenotypes
2025
December 2025
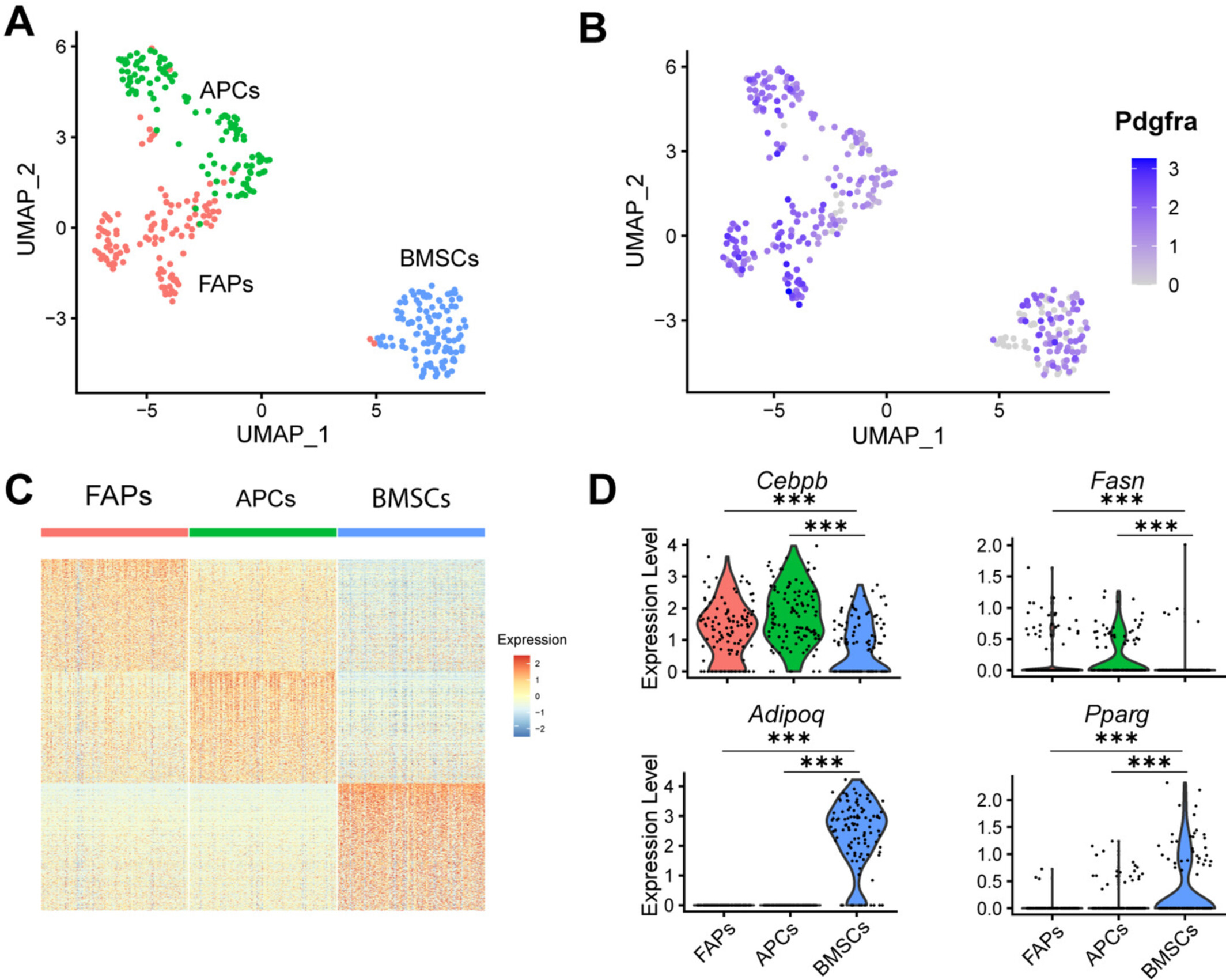
Tissue-specific effects of senescence on adipogenic potential
This collaborative study, published in the journal Obesity, outlines the differential effects of senescence on adipogenesis in mesenchymal progenitors from bone, muscle, and adipose tissue. Interestingly, senescence inhibits adipogenesis in progenitors from fat and muscle, but inhibits adipogenesis in progenitors from bone, suggesting a possible mechanism contributing to age-related alterations in tissue adiposity.
Senescent Mesenchymal Stromal Cells Differentially Alter Adipogenesis in Adipose Tissue, Skeletal Muscle, and Bone Marrow
September 2025

Geordie joins the lab!
Geordie Emberling joins the lab as a rotation Ph.D. student. Welcome Geordie!
September 2025

Lesley joins the lab!
Lesley Rendon-Hernández joins the lab as a research technician. Welcome Lesley!
September 2025

Joseph joins the lab!
Joseph Maye joins the lab as a research technician. Welcome Joe!
July 2025

Dr. Doolittle Named a 2025 UConn Pepper Scholar
This award from the Research Education Component (REC) of the NIH-funded UConn Claude D. Pepper Older Americans Independence Center provides funding and career development activities related to research on geriatrics and gerontology. More about this story and Pepper Center Homepage.
July 2025

Doolittle Lab Receives AFAR Junior Faculty Grant
This funding from the American Federation for Aging Research (AFAR) will support the project on premature aging and residual senescence after injury resolution; a new direction understanding long-term effects of fractures on skeletal aging and senescence. More about this story
June 2025

Doolittle Lab Receives Funding From the UConn Health Research Excellence Program
Dr. Madison Doolittle received funding from the UConn Health Research Excellence Program (REP) Convergence Grant for a collaborative project with Drs. Beiyan Zhou and David Rowe. This work will test candidate factors regulating senescent cell immune modulation through novel approaches.
May 2025

Dr. Doolittle Receives Dr. Marion Frank Faculty Development Award
Dr. Madison Doolittle received this award from the UConn School of Dental Medicine to pursue individual career development activities. This will fund the optimization of single nucleus isolation from mineralized tissues. More about this story.
2024
September 2024

Dr. Doolittle Wins Most Outstanding Translational Abstract Award at ASBMR 2024
Dr. Madison Doolittle received this award from President Dr. Laura Calvi at the 2024 annual meeting of the American Society for Bone and Mineral Research (ASBMR) for his abstract "Aged Immune Cells Do Not Exhibit Chronic Senescence Akin to Mesenchymal-Lineage Cells in Bone." This work was performed while under the mentorship of Dr. Sundeep Khosla at Mayo Clinic and couldn't have been done without the mentorship and support of Dr. Khosla and his laboratory.
June 2024

Collaborative SenNet Study Online in Nature Reviews Molecular Cell Biology
Comprehensive paper by the SenNet Consortium outlining the defining characteristics of senescent cells in various tissues. SenNet recommendations for detecting senescent cells in different tissues | Nature Reviews Molecular Cell Biology
June 2024


Li Chen and Zhihua (Lisa) Wu Join the Doolittle Lab!
Welcome to Li and Lisa! Looking forward to working with you both.
June 2024

Fracture Senescence Paper Is Online in the Journal of Clinical Investigation
New study in outlining the expression of senescence markers in healing fracture callus at the single-cell level: Osteochondroprogenitor cells and neutrophils expressing p21 and senescence markers modulate fracture repair. Dr. Doolittle served as co-first author and co-corresponding author.
May 2024
